Abstract
The F1 and V antigens of Yersinia pestis, despite acting as virulence factors secreted by the organism during infection, also combine to produce an effective recombinant vaccine against plague, currently in clinical trial. The protective mechanisms induced by rF1 + rV probably involve interactions with dendritic cells (DC) as antigen uptake, processing and presenting cells. To study such interactions, naive ex vivo DC from bone marrow, spleen and lymph node were cultured with rF1, rV or combined antigens and demonstrated to secrete interleukin (IL)-4 and IL-12 into the culture supernatant. Cytokine production in response to pulsing was dependent on the maturity of the bone marrow-derived DC culture, so that pulsed 8-day-old cultures had accumulated significantly more intracellular IL-4 and IL-12 than unpulsed cells. DC, pulsed with rF1 + rV for 2–24 h, were able to prime naive autologous lymph node T cells to proliferate in an antigen dose-dependent manner, with an order of potency of 3d bone marrow-derived DC (BMDC) > 7d BMDC > splenic DC. Significantly, cell-free supernatants from rF1 + rV-pulsed BMDC and splenic DC were also able to induce specific primary responses effectively in naive T cells, suggesting that these supernatants contained stimulatory factor(s). This study suggests an important role for DC, or factors secreted by them, in the induction of protective immunity to plague by the rF1 and rV antigens.
Keywords: dendritic cells, rF1, rV, Yersinia pestis
Introduction
The virulence mechanisms exerted by the plague-causing bacterium, Yersinia pestis, are complex and have evolved with the changing lifestyle of the organism [1,2]. The result is that Y. pestis is one of the most virulent bacterial pathogens known. While other members of the Yersinia genus (Y. pseudotuberculosis and Y. enterocolitica) cause mild but self-limiting infections in man [3], Y. pestis can cause a lethal infection if undetected, undiagnosed and untreated [2]. This is intriguing, given the recent evidence that Y. pestis evolved away from the lifestyle of Y. pseudotuberculosis estimated between 1500 and 20 000 years ago [1], to become infective systemically rather than perorally. As a result of this evolutionary divergence, Y. pestis may have acquired some virulence mechanisms which differ from those of the other Yersiniae and which are more suited to its current lifestyle [4]. The virulence of Y. pestis is determined largely by three major plasmids which it carries: the 100–110 kb pFra, the 70kb pCD1 and the 9.5kb pesticin plasmid (pPCP1). The pFra and pPcP1 plasmids are present only in Y. pestis[3]. While the pFra plasmid shows extensive sequence homology with a plasmid (pHCM2), possessed by some strains of Salmonella enterica serovar Typhi[5], some regions of pFra appear to be unique to Y. pestis. One of these regions includes the caf operon encoding the 17 kDa polypeptide Fraction 1 (F1) capsular antigen [6] which has anti-phagocytic activity. Plasmid CD1 (whose homologue in Y. enterocolitica and Y. pseudotuberculosis is pYV) encodes a type III secretion system (TTSS) which comprises a secretion apparatus, chaperones, several secreted effector proteins including the low calcium response V antigen (lcrV) and a series of yersinia outer proteins (Yops) (for review see References [7–10]). The lcrV protein is thought to play a key role in regulating the TTSS in all the yersinia species, both intracellularly and at the cell surface [11], and is intrinsic to forming a channel between the bacterium and the host cell, through which the Yop proteins can be translocated [7]. This translocation channel comprises the protein YscF and the lcrV protein has been identified at the distal tip [12], possibly functioning to make contact with the host cell. The translocated Yops have potent anti-host functions, with YopM thought to cause an early and severe depletion of natural killer (NK) cells, which are important in the host's innate immune defence [13,14]. Other Yops, such as YopJ (YopP in Y. enterocolitica), are thought to act by reducing the display of cellular activation markers on immune effector cells, which in turn damps down the induction of proinflammatory cytokines such as tumour necrosis factor (TNF)-α and may be implicated in the induction of apoptosis in infected cells [15,16], although YopJ may be less effective than YopP in this, due to poor translocation from Y. pestis to the target cell [17]. Although this effect of Yop P has been observed for Y. enterocolitica, paradoxically the activation marker CD54 has been observed on dendritic cells during Y. pestis infection [18]. Hence, it is thought that reducing the display of cellular activation markers is not the principal mechanism used by Y. pestis to disarm dendritic cells (DC). Instead, Y. pestis may have the effect of reducing the maturation and adhesion of DC for endothelial surfaces and therefore reduces their ability to migrate to the site of infection [18]. There is evidence that Y. pestis preferentially hijacks monocytes or macrophages and uses them as a safe niche in which to multiply [19,20], and may deter other antigen-presenting cells (APC) such as DC, so that the major classes of APC are functionally neutralized. In this situation, antigen presentation to T cells would not occur and no immune response would be initiated.
DC are arguably the most potent APC known [21], and have a surveillance function for invading microorganisms or microbial products. Contact with the microorganism triggers DC maturation with uptake, internalization and processing of the pathogen, accompanied by cellular signalling initiated by Toll-like receptor recognition [22–26].
In this context, it was of interest to determine the direct effect on DC of the F1 and V protein antigens of Y. pestis, particularly as these have a dichotomous role: as purified recombinant proteins combined in a vaccine they are potently immunogenic and protective against plague infection [27–29]; on the other hand, each of these proteins functions as a potent virulence factor with an anti-phagocytic (F1) and a pivotal regulatory role in TTSS(V), respectively, when expressed by the organism during infection [2]. The ability of the ex vivo DC to interact with the F1 and V antigens and to initiate a primary immune response in naive T cells in vitro has been studied. The F1 and V antigens stimulated the production of both type 1- and type 2-promoting cytokines from the DC and cell-free supernatants from the pulsed DC were also able to induce proliferation in naive T cells.
Materials and methods
Animals
Female Balb/c mice between 6 and 8 weeks old (Harlan UK, Bicester, UK) were purchased as specific pathogen free (SPF) stock and housed in groups of five with free access to food, water and environmental enrichment.
Antigens
Recombinant proteins V and F1 were used. The V antigen was expressed as a fusion protein with glutathione-S-transferase (GST) in Escherichia coli and then cleaved and purified [30]. The F1 antigen was expressed from the Y. pestis caf operon, cloned in E. coli and purified from the supernatant [31]. Both proteins were treated with immobilized polymyxin B to remove lipopolysaccharide (LPS) and the absence of LPS was confirmed by assaying the proteins in the limulus amoebocyte lysate (LAL) assay (Charles River Endosafe, Charleston, SC, USA).
Isolation of dendritic cells from spleen
Single-cell suspensions were prepared from mouse spleen by pressing through a sterile wire gauze into complete medium comprising RPMI-1640 Dutch modification (Sigma-Aldrich, Poole, UK) supplemented with 10% fetal calf serum, 100 U/ml penicillin, 100 µg/ml streptomycin, 2 mM glutamine and 5 × 10−5 M 2-mercaptoethanol. Cells were incubated in T25 tissue culture flasks (Falcon, Cockeysville, MD, USA) overnight and non-adherent splenocytes (5 ml) layered onto 2 ml of metrizamide (analytical grade 13.7% w/v; Nygaard, Oslo, Norway) and centrifuged at 600 g for 10 min at room temperature. Low-density interface cells were collected, washed once and resuspended in medium; >70% were DC by morphology and displayed CD11c [32].
Growth of dendritic cells from bone marrow
Bone marrow cells from mouse femurs were flushed into complete medium, washed once and the single-cell suspension overlaid onto lympholyte M (Cedarlane, Ontario, Canada) and centrifuged at 1200 g for 30 min at room temperature. The interface cells were washed and cultured at 1 × 106 cells/ml in complete medium supplemented with granulocyte–macrophage colony-stimulating factor (GM-CSF) (100 U/ml) and TNF-α (50 U/ml). On day 3, non-adherent cells were washed and either centrifuged over metrizamide and DC at the interface washed and analysed, or replaced in the original tissue culture flask with complete medium supplemented with GM-CSF and TNF-α (50 U/ml). After 6–14 days' culture, non-adherent cells were centrifuged over metrizamide, as described above. Cells were > 95% viable (Trypan blue) and from light scatter and phenotype were > 95% DC [33].
Measurement of cytokines
DC culture supernatants were assayed by OptEIATM mouse interleukin (IL)-4, IL-10, IL-12 (p40), interferon (IFN)-γ set (Pharmingen, San Diego, CA, USA). Nunc-ImmuneTM 96-well microtitre plates (Nalge Nunc International, Roshilde Denmark) were coated with the appropriate capture monoclonal antibody (mAb). Cytokine binding was detected with biotinylated detection antibody and avidin–horseradish peroxidase conjugate. The data are presented as mean concentrations of specific cytokines (pg/ml) with standard errors.
Intracellular cytokine measurements
DC in medium, some with 3 mM monensin (Calbiochem, San Diego, CA, USA), were incubated at 37°C for 6 h, washed twice and fixed for 15 min in solution A of Leucoperm kit (Serotec, Kidlington, Oxford, UK). Following one wash, DC were resuspended in permeabilization medium (solution B of Leucoperm kit), rabbit serum and rat anti-mouse IL-10 phycoerythrin (PE), rat anti-mouse IL-12p70 PE or rat anti-mouse IL-4 PE antibody and incubated in the dark at room temperature for 30 min. Cells were washed, fixed in 1% paraformaldehyde, stored at 2–8°C in the dark and analysed by flow cytometry within 24 h. Analysis was carried out using the winlist software program (Verity Software, Topsham, ME, USA) with calculation of positive cells (%) by enhanced normalized subtraction (ENS) where the histograms of the control (no monensin) and the test (with monensin) samples are compared and subtraction used to assess positivity. The test sample is represented by an open histogram and the number of positive events (%) by the filled area within this histogram. Negative ENS results are recorded as 0% values. Kolmogorov–Smirnov statistics were used to assess the significance of a positive result by calculating the critical Dvalue (Dcrit) with the following equation:
where Dmax is the maximum value between the test (with monensin) and control (no monensin) samples after the two histograms have been converted into cumulative normalized histograms, n1 is the number of events in the test sample and n2 is the number of events in the control sample. Dcrit with P < 0.01 was accepted as a significant positive value. Student's paired t-test was used for assessing the differences between DC unpulsed or pulsed with rV and rF1 antigens at days 3, 8 and 13 of culture.
Flow cytometry
Cells in cold phosphate-buffered saline (PBS) containing ethylenediamine tetraacetic acid (EDTA) (1 mM), sodium azide (0.02%) and fetal calf serum (2%) were incubated on ice with fluorescein isothiocyanate (FITC)-conjugated mouse antibodies H-2Dd (mouse IgG2a, clone 34-2-12); H-2Kd (mouse IgG2a); H-2Dk (mouse IgG2a clone 15-5-55); H-2Kk (mouse IgG2a); H-2Ad (mouse IgG2b, clone AMS-32.1); H-2Ak (IgG2b, clone 11-5-2); H-2Ek (mouse IgG2a, clone 17-3-3); CD11c (clone HL3) and IgG (clone HL3). Isotype-control antibodies were FITC-conjugated mouse IgG2a, IgG2b, hamster IgG (Pharmingen).
Primary proliferative responses
Dendritic cells (500, 1000 and 2000/well) either unpulsed or pulsed for 2–24 h with rV, rF1, a combination of both antigens or V peptides, were washed and cultured with (25–100) × 103 T cells, taken from inguinal, brachial and axillary lymph nodes, in triplicate 20 µl drops in Terasaki plates. Control responses were obtained using concanavalin A stimulation (5 µg/ml). Plates were inverted and cultured for 3 or 4 days over sterile saline in plastic boxes at 37°C. Each hanging drop received 1 µl [3H]-thymidine (74 GBq/mM, 1 µg thymidine/ml; GE Healthcare, Amersham, UK) and after 2 h at 37°C they were blotted onto filter discs, washed with saline, trichloroacetic acid (5%) and methanol and counted in a scintillation counter [32].
Preparation of supernatants
DC from spleen cultures were pulsed with rV (10 µg/ml) or rF1 (20 µg/ml) and 3-day bone marrow-derived DC (BMDC) were pulsed with rV (10 µg/ml) or rF1 (5 µg/ml) for 2–4 h, washed twice by centrifuging them at 400 g for 10 min and cultured overnight at a concentration of 1–2 × 105 DC/0.1 ml medium in round-bottomed tubes. The tubes were centrifuged at 10 000 g for 10 min and supernatant transferred to sterile Eppendorf tubes and microfuged at 10 000 g for 15 min to remove cell debris. Supernatant (1–3 µl) was used to stimulate (40–160) × 103 syngeneic lymph node T cells/well, in triplicate 20 µl cultures. Proliferation was measured on day 3 of culture as described above.
Results
Cytokine secretion by dendritic cells
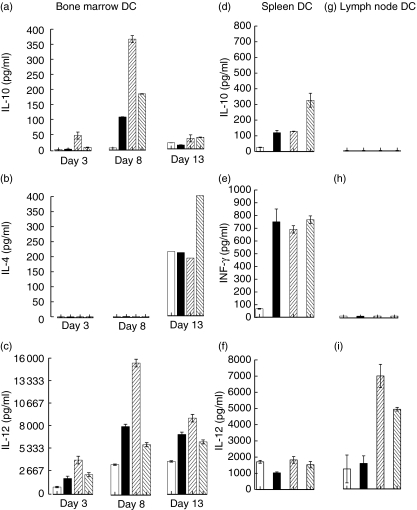
Initially, the cytokine response of DC from three different sources (bone marrow, spleen and lymph node) to ex vivo pulsing with single or combined antigens (rF1, rV or rF1 + rV) was assessed (Fig. 1). BMDC samples were subdivided into 3-, 8- or 13-day-old cultures prior to pulsing with antigens, while splenic and lymph node DC were used without ageing. BMDC (Fig. 1a–c) and splenic DC (Fig. 1d–f) cultures produced the cytokines IL-10, IL-4, IL-12 and IFN-γ. There was some differential production; for example, day 8 BMDC produced most IL-10 and IL-12, both of which were up-regulated by pulsing the DC with rF1 and rV, whereas significant quantities of IL-4 were detected only from day 13 BMDC and this was up-regulated by the combined antigens. Splenic DC produced IL-12 (which was not up-regulated by rF1 and rV) and IFN-γ (which was up-regulated by pulsing with the antigens). IL-12 only was detected in the supernatant from lymph node DC (Fig. 1g–i), but this was up-regulated in response to rF1 and rF1 + rV. Thus the timing and quantity of cytokines produced varied with the cell source and maturity.
Fig. 1.
Production of cytokines by dendritic cells (DC). Bone marrow, spleen and lymph node-derived DC were either unpulsed (open box) or pulsed with rV (solid box), rF1 (/hatched box), rV + rF1 (\hatched box) for 2–24 h. Supernatants were collected and assayed by enzyme-linked immunosorbent assay (ELISA) (a,b,c). Production of interleukin (IL)-10 (a); IL-4 (b); IL-12 (c) in 3-, 8- and 13-day-old bone marrow-derived DC (BMDC) are shown from triplicate experiments except for the day 13 IL-4. No IL-4 was seen in 3- and 8- and only intermittently in 13-day-old BMDC and the result from one positive experiment of three is shown (b). Production of IL-10 (d); interferon (IFN)-γ (e) and IL-12 (f) in splenic (d–f) and lymph node DC (g–i).
Intracellular cytokine production
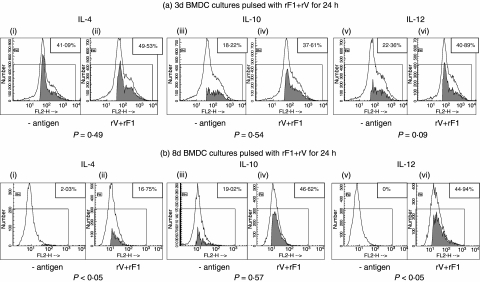
Due to the differential production of cytokines with age of BMDC, further analysis of intracellular cytokines in BMDC was undertaken. For this, 3-, 8- or 13-day cultures of BMDC were pulsed with the combined rF1 + rV antigens for 24 h prior to the assay of intracellular IL-4, IL-10 and IL-12 (Fig. 2). Production of cytokine within the cells, both with and without exposure to antigen, was measured by its accumulation following exposure of the cell for 6 h to monensin. Monensin itself did not stimulate cytokine production and specificity of the labelling was confirmed in early experiments using these reagents by demonstrating blocking with excess specific cytokine (not shown). Cytokine expression was either up-regulated or initiated by exposure to rV + rF1. DC showed background production of each cytokine in a proportion of the experiments. However, the maturity of the cells was again critical for the production of cytokines within the BMDC. Figure 2 shows cytokine production in a representative experiment. The shaded areas within the histograms represent the percentages of positive events, after winlist ENS calculations, and these values are shown in the boxed areas. Plots of DC treated with rV + rF1 (Fig. 2a,ii,iv,vi; Fig. 2b,ii,iv,vi) were greater than those not treated with antigen (Fig. 2a,i,iii,v; Fig. 2b,i,iii,v). Data from three to four experiments of this kind are summarized in Fig. 3. The intracellular IL-4 was seen only in the presence of monensin, indicating that it was being produced within the cells and not remaining from their IL-4 exposure during development. We showed, in parallel studies, that the presence of intracellular IL-4 was promoted by the exposure of the developing DC to IL-4 through binding to IL-4 receptors on DC [34]. The up-regulatory effect of the antigens on day 8 BMDC was significant over all experiments for IL-4 (Fig. 3a) and IL-12 (Fig. 3c). For day 13 BMDC, there was a significant reduction of IL-4 and IL-10 in one of three individual experiments but these effects were variable and not significant overall. The production of IL-10 was seen reproducibly only when the cells were pre-exposed to antigen for 24 h before the block with monensin, whereas IL-4 and IL-12 production were seen following a 1-h exposure to antigen before the addition of the monensin. Thus, pulsing the BMDC with the combined antigens induced both IL-12 and IL-4 production in parallel in the same cell populations.
Fig. 2.
Production of intracellular interleukin (IL)-4, IL-10 and IL-12 cytokines in bone marrow-derived dendritic cells (BMDC). BMDC were either unpulsed or pulsed with rV (10 µg/ml) + rF1 (5 µg/ml) for 24 h. BMDC were taken from cultures at 3 days (a) or 8 days (b). The open histogram represents the test sample with monensin; the shaded area shows the positive percentage of events when the control sample (no monensin) is compared and subtracted from the test sample. The boxed figures show the enhanced normalized subtraction (ENS) percentages of these positive values. Kolmogorov–Smirnov statistical analysis was used to assess the significance of this positivity. Production of IL-4, IL-10 and IL-12 were significant (P < 0.01). At day 13 (not shown) only the increase of IL-12 obtained in pulsed BMDC was significant (P < 0.01).
Fig. 3.
Production of intracellular cytokines in bone marrow-derived dendritic cells (BMDC). The percentage of BMDC positive for interleukin (IL)-4 (a), IL-10 (b) or IL-12 (c), represented as the mean ± standard error of the mean (s.e.m.) obtained from three to four experiments of the type shown in Fig. 4, are shown for days 3, 8 and 13 BMDC. BMDC were either unpulsed or pulsed with rV + rF1 (peps) for 24 h. Paired t-test statistical analysis was used. The increases of IL-4 and IL-12 were significant overall (P < 0.05) at day 8.
Primary stimulation with antigen-pulsed DC
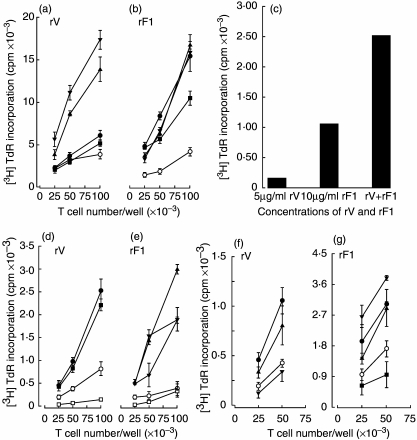
Next, the ability of rF1- or rV-pulsed BMDC and splenic DC to prime naive syngeneic lymph node T cells was assessed by pulsing with tritiated thymidine (Fig. 4). Freely available thymidine of low specific activity for a short 2-h pulse time was used to give uptakes that truly reflected DNA syntheses with minimal radiation damage to cells taking up the tritium [35]. For reference, responses to concanavalin A, the positive control used in all experiments, ranged between 5086 and 26 800 counts per min (cpm). Both BMDC and splenic (gradient-separated) DC pulsed with antigen were used in these studies; the BMDC were derived from stem cells by growth in the presence of GM-CSF and IL-4 and studied between days 3 and 13 of culture. BMDC from 3-day cultures, which had been pulsed for 18 h with the antigens, stimulated high levels of primary T cell proliferation (Fig. 4a,b) and were more effective than splenic DC which had been pulsed for 18 h with single or combined antigens (Fig. 4c) in stimulating proliferation; pulsing doses of 1–10 µg/ml of rV or 0.5–10 µg/ml of rF1 were optimal. Seven-day-old BMDC (Fig. 4d,e) pulsed for 2 h with rV or rF1 stimulated lower primary proliferative responses, which were dose-dependent for rV. Higher antigen pulsing doses (10–20 µg/ml of rV antigen for 2 h) or (20–50 µg/ml of rF1 for 4 h) were required to achieve primary proliferation of naive T cells with splenic DC (Fig. 4f,g).
Fig. 4.
Primary T cell proliferative responses in vitro to rV and rF1 antigens. Different numbers of lymph node T cells from naive mice were cultured with 1000 autologous dendritic cells (DC), which were either unpulsed or pulsed with antigens. Proliferation was measured on day 3 of culture. (a, b) Three-day bone marrow-derived DC (BMDC) were either unpulsed (O) or pulsed with rV (a) or rF1 (b) at 0.1 (▪), 0.5 (•), 5 (▴), 10 (▾) for 18 h. (c) Naive donor T cells (105) were cultured with splenic DC pulsed with 5 µ g/ml rV, 10 µg/ml rF1 or both antigens at these same concentrations for 18 h. (d–e) Seven-day BMDC. Responder cells only (□). DC were either unpulsed (O) or pulsed with rV (c) at 5 (▪) and 10 µg/ml (•) or rF1 (d) at 1 (▴), 5 (▾) and 10 µg/ml (★) for 2 h. (f–g) Splenic DC were either unpulsed (O) or pulsed with rV (e) at 2.5 (▾), 4.5 (▴) and 10 µg/ml (•) for 2 h; or rF1 (f) at 5 (▪), 10 (•), 20 (▴) and 25 µg/ml (▾) for 4 h. All figures are representative of data gained in at least three experiments.
Primary proliferative responses in T cells exposed to supernatants from antigen-pulsed DC
Because antigen-pulsed 3d BMDC were most stimulatory for naive T cells, cell free supernatants were collected from these cultures which had been pulsed for 4 h with rF1 or rV and then washed and incubated overnight. Initially, 1 µl of supernatant was cultured with naive syngeneic lymph node T cells for 3–4 days and thymidine uptake into new DNA measured. A significant response to the supernatant from rF1- and rV-pulsed BMDC was obtained (Fig. 5a). A significant proliferative response in naive T cells was also induced by the supernatant from rF1-pulsed, but not rV-pulsed, splenic DC (Fig. 5b), and increasing the volume of stimulatory supernatant used to 3 µl increased responder cell proliferation to levels equivalent to those induced by 3d BMDC (Fig. 5c). More mature BMDC (from 6- to 12-day cultures) did not produce stimulatory supernatants (not shown). If the antigen-pulsing period was increased from 2–4 h to 6–24 h, the supernatants were not stimulatory.
Fig. 5.
Primary T cell proliferative responses to supernatants from rV- and rF1-pulsed DC. (a) Cell free supernatants were collected at 24 h of culture from 3d bone marrow-derived DC (BMDC) which had been pulsed with rV and rF1 for 4 h, and then washed. The supernatants (1 µl) were used to stimulate syngeneic lymph node T cells. Lymph node cells alone (□); supernatants from unpulsed DC (O); supernatants from rV- and rF1-pulsed DC (▴) and (▾), respectively. (b, c) Cell free supernatant (b: 1 µl, c: 3 µl) from splenic DC pulsed for 2 h with rV and rF1 and used to stimulate autologous lymph node T cells. Legends for data sets are represented as in (a).
By comparison, supernatants from rV-pulsed splenic DC caused marginal effects that were only 1.5–2 times the background and when the pulse time with antigen increased to 24 h, less stimulation was seen (not shown). Taken together, these data suggest that the stimulatory component(s) in DC supernatants was produced early after the exposure to antigen.
Discussion
This study has demonstrated that the in vitro interaction of DC with the rF1 and rV proteins leads to the induction of primary proliferative responses in lymph node T cells and the secretion of a spectrum of cytokines into the cell culture supernatant. While DC derived from peripheral lymph nodes principally secreted IL-12 when pulsed with rF1 + rV, and appeared to have a type 1 default cytokine pattern, DC from systemic sources (spleen and bone marrow) secreted the range of types 1 and 2 cytokines. It was also observed that a 24-h pulse with rF1 and rV in vitro, accelerated the expression of major histocompatibility complex (MHC) molecules on 15–20% of the BMDC taken at 3–9 days of culture and hence the maturation of these cells (data not shown), although such effects were difficult to quantify because of the biphasic nature of MHC class II expression on the cells as they matured.
Intracellular cytokine production in BMDC was studied by flow cytometry. This measurement was particularly important for IL-4, where IL-4 binding back onto its own receptor on DC means that little secreted cytokine is detected [34]; a similar situation has been described for other cell types [36]. In our studies, secreted IL-4 was detected only at day 13 of culture and with DC exposed to antigens for 24 h but, as expected, IL-4 was demonstrated more readily by intracellular analysis. The production of IL-4 seen in unstimulated cells is also likely to be a consequence of the exposure to IL-4 during the development of the DC [34]. There were variations depending on the maturity of the cells, but using 8-day cells there was up-regulation of intracellular IL-12 and IL-4. The duration of exposure to the antigens required to stimulate cytokine production varied. Thus IL-10 and IL-4 were induced after 24 h exposure to the antigens before the block with monensin, whereas IL-12 was induced after only 1 h of exposure to the antigens. Although these variations added to the problems of assessing the effects of these antigens on cytokine production so that the use of multiple time-points both in the maturation of DC and in exposure to antigen were required, it appeared that the processing of rF1 + rV by DC resulted in the production of both type 1 and type 2 cytokines. This differs from the processing of other microbial products, such as Leishmania major promastigotes, by DC which results in polarization towards a type 1 response with IL-12 secretion [23].
Significantly, the cell-free culture supernatants from rF1-pulsed DC, were also able to induce primary T cell stimulation, due presumably to the secretion of stimulatory factor(s) secreted by the DC. The most effective production of such material was when the DC were exposed to antigens for just a short period (2–4 h) and the stimulatory products were secreted over the next 24 h. In addition, immature DC derived from the bone marrow (3d BMC) were more effective at secreting stimulatory factors than DC from other sources.
It is known that when DC are pulsed with antigens to induce primary responses, the antigen is transferred to other DC [22,32,37,38]. If these DC secondarily acquiring antigen have not already been exposed directly to antigen, they stimulate a primary T cell response. This requirement for transfer of antigen between DC has been seen in primary stimulation of allogeneic cells, and following exposure of DC to contact sensitizer, viral antigens and peptides [37,39,40]. In addition, in vivo, DC of recipient animals may be required for production and/or amplification of primary immune responses to antigens delivered by DC or from DC-derived exosomes [41–45]. DC secrete a high proportion of their MHC class II molecules into the supernatant in the form of exosomes [46]. Such exosomes also contain heat shock protein, which may act as an adjuvant. Although electron microscope pictures suggest that DC interact with other DC or monocytes by direct contact [47] and may transfer antigen in this way, exosomes or secreted factors may also be a major route of antigen transfer. Evidence supporting this view is that the supernatants of DC exposed to a variety of antigens induce primary T cell responses in vitro if the responding lymphocyte populations contain DC [32,37]. Exosomes from DC have previously been used successfully to vaccinate mice against tumours [48] and such factors may also constitute a putative novel generic approach to anti-microbial vaccination. In future experiments we plan to characterize these DC products, as they have the potential advantage of harnessing the potency of DC without the problems associated with using live cell vaccines.
Proof of principle that DC can induce protective immunity by passive transfer has already been gained from studies in which immune naive mice received GM-CSF + TNF-α-matured BMDC which had also been pulsed with heat-killed Burkholderia pseudomallei[49] and developed both cellular and antibody responses to the pathogen [50].
The data gained in this study indicate that the interaction of DC with the rF1 and rV protein antigens of Y. pestis has induced an adaptive primary immune response in naive lymph node T cells in vitro. The in vivo processing of the rF1 + rV antigens and subsequent presentation to T cells are critical components of the success of this antigen combination in the observed protective immunity induced by this next-generation plague vaccine [28]. These effects are in marked contrast to those of infection with the whole organism Y. pestis on DC function, which are to reduce DC adhesion and migration to the site of infection [18]. These are due probably to integrated virulence mechanisms exerted by this complex organism which inhibit the initiation of processing and presentation of Y. pestis antigens such as F1 and V in vivo and thus prevent the establishment of a protective immune response. However, we speculate that selection of multiple primary T cell epitopes stimulating both type 1 and type 2 Th cell-promoting cytokines in DC may be characteristic of a vaccine successful in stimulating both cellular and humoral immunity effective against the intracellular and extracellular lifecycle phases of Y. pestis.
References
- 1.Achtman M, Zurth K, Morelli G, Torrea G, Guiyoule A, Carniel E. Yersinia pestis, the cause of plague, is a recently emerged clone of Yersinia pseudotuberculosis. Proc Natl Acad Sci USA. 1999;96:14043–8. doi: 10.1073/pnas.96.24.14043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perry RD, Fetherston JD. Yersinia pestis – etiologic agent of plague. Clin Microbiol Rev. 1997;10:35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brubaker RR. Factors promoting acute and chronic diseases caused by yersiniae. Clin Microbiol Rev. 1991;4:309–24. doi: 10.1128/cmr.4.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parkhill J, Wren BW, Thomson NR, et al. Genome sequence of Yersinia pestis, the causative agent of plague. Nature. 2001;413:523–7. doi: 10.1038/35097083. [DOI] [PubMed] [Google Scholar]
- 5.Prentice MB, James KD, Parkhill J, et al. Yersinia pestis pFra shows biovar-specific differences and recent common ancestry with a Salmonella enterica serovar Typhi plasmid. J Bacteriol. 2001;183:2586–94. doi: 10.1128/JB.183.8.2586-2594.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galyov EE, Smirnov OY, Karlishev AV, et al. Nucleotide sequence of the Yersinia pestis gene encoding F1 antigen and the primary structure of the protein. FEBS Lett. 1990;277:230–2. doi: 10.1016/0014-5793(90)80852-a. [DOI] [PubMed] [Google Scholar]
- 7.Cornelis GR. The yersinia Ysc–Yop ‘type III’ weaponry. Nat Rev Mol Cell Biol. 2002;3:742–52. doi: 10.1038/nrm932. [DOI] [PubMed] [Google Scholar]
- 8.Cornelis GR, Wolf-Watz H. The yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol Microbiol. 1997;23:861–7. doi: 10.1046/j.1365-2958.1997.2731623.x. [DOI] [PubMed] [Google Scholar]
- 9.Mota LJ, Cornelis GR. The bacterial injection kit: type III secretion systems. Ann Med. 2005;37:234–49. doi: 10.1080/07853890510037329. [DOI] [PubMed] [Google Scholar]
- 10.Viboud GI, Bliska JB. Yersinia outer proteins: role in modulation of host cell signalling responses and pathogenesis. Annu Rev Microbiol. 2005;59:69–89. doi: 10.1146/annurev.micro.59.030804.121320. [DOI] [PubMed] [Google Scholar]
- 11.Pettersson J, Holmstrom A, Hill J, et al. The V-antigen of Yersinia is surface exposed before target cell contact and involved in virulence protein translocation. Mol Microbiol. 1999;32:961–76. doi: 10.1046/j.1365-2958.1999.01408.x. [DOI] [PubMed] [Google Scholar]
- 12.Mueller CA, Broz P, Muller SA, et al. The V-antigens of Yersinia forms a distinct structure at the tip of injectisome needles. Science. 2005;310:674–6. doi: 10.1126/science.1118476. [DOI] [PubMed] [Google Scholar]
- 13.Congleton YHK, Wulff CR, Kerschen EJ, Straley SC. Mice naturally resistant to Yersinia pestisΔpgm strains commonly used in pathogenicity studies. Infect Immun. 2006;74:6501–4. doi: 10.1128/IAI.00597-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerschen EJ, Cohen DA, Kaplan AM, Straley SC. The plague virulence protein YopM targets the innate immune response by causing a global depletion of NK cells. Infect Immun. 2004;72:4589–602. doi: 10.1128/IAI.72.8.4589-4602.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palmer LE, Hobbie S, Galan JE, Bliska JB. YopJ of Yersinia pseudotuberculosis is required for the inhibition of macrophage TNF-α production and downregulation of the MAP kinases p38 and JNK. Mol Microbiol. 1998;27:953–65. doi: 10.1046/j.1365-2958.1998.00740.x. [DOI] [PubMed] [Google Scholar]
- 16.Ruckdsechel K, Harb S, Rogegnkamp A, et al. Yersinia enterocolitica impairs activation of transcription factor NF-kappa B: involvement in the induction of programmed cell death and in the suppression of the macrophage tumour necrosis factor alpha production. J Exp Med. 1998;187:1069–79. doi: 10.1084/jem.187.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zauberman A, Cohen S, Mamroud E, et al. Interaction of Yersinia pestis with macrophages: limitations in YopJ-dependent apoptosis. Infect Immun. 2006;74:3239–50. doi: 10.1128/IAI.00097-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Velan B, Bar-Haim E, Zauberman A, Mamroud E, Shafferman A, Cohen S. Discordance in the effects of Yersinia pestis on the dendritic cell functions manifested by induction of maturation and paralysis of migration. Infect Immun. 2006;74:6365–76. doi: 10.1128/IAI.00974-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lukaszewski RA, Kenny DJ, Taylor RDGC, Rees Hartley MG, Oyston PCF. Pathogenesis of Yersinia pestis infection in Balb/c mice: effects on host macrophages and neutrophils. Infect Immun. 2005;73:7142–50. doi: 10.1128/IAI.73.11.7142-7150.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marketon MM, DePaolo RW, DeBord KL, Jabri B, Schneewind O. Plague bacteria target immune cells during infection. Science. 2005;309:1739–41. doi: 10.1126/science.1114580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 22.Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20:561–7. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 23.Konecny P, Stagg AJ, Jebbari H, English N, Davidson RN, Knight SC. Murine dendritic cells internalize Leishmania major promastigotes, produce IL-12 p40 and stimulate primary T cell proliferation in vitro. Eur J Immunol. 1999;29:1803–11. doi: 10.1002/(SICI)1521-4141(199906)29:06<1803::AID-IMMU1803>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 24.Macatonia SE, Taylor PM, Knight SC, Askonas BA. Primary stimulation by dendritic cells induces antiviral proliferative and cytotoxic T cell responses in vitro. J Exp Med. 1989;169:1255–64. doi: 10.1084/jem.169.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.d'Ostiani CF, Del Sero G, Bacci A, et al. Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans. Implications for initiation of T helper cell immunity in vitro and in vivo. J Exp Med. 2000;191:1661–74. doi: 10.1084/jem.191.10.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sousa CR, Hieny S, Scharton-Kersten T, et al. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J Exp Med. 1997;186:1819–29. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powell BS, Andrews GP, Enama JT, et al. Design and testing for a non-tagged F1–V fusion protein as vaccine antigen against bubonic and pneumonic plague. Biotechnol Prog. 2005;21:1490–510. doi: 10.1021/bp050098r. [DOI] [PubMed] [Google Scholar]
- 28.Williamson ED, Flick-Smith HC, Lebutt CS, et al. Human immune response to a plague vaccine comprising recombinant F1 and rV antigens. Infect Immun. 2005;73:3598–608. doi: 10.1128/IAI.73.6.3598-3608.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williamson ED. Plague vaccine research and development. J Appl Micro. 2001;91:606–8. doi: 10.1046/j.1365-2672.2001.01497.x. [DOI] [PubMed] [Google Scholar]
- 30.Carr S, Miller J, Leary SEC, Bennett AM, Ho A, Williamson ED. 2000. Expression of a recombinant form of the V antigen of Yersinia pestis, using three different expression systems. Vaccine. 2000;18:153–9. doi: 10.1016/s0264-410x(99)00214-5. [DOI] [PubMed] [Google Scholar]
- 31.Jones SM, Griffin KF, Hodgson I, Williamson ED. Protective efficacy of a fully recombinant plague vaccine in the guinea pig. Vaccine. 2003;21:3912–8. doi: 10.1016/s0264-410x(03)00379-7. [DOI] [PubMed] [Google Scholar]
- 32.Knight SC, Iqball S, Roberts MS, Macatonia S, Bedford PA. Transfer of antigen between dendritic cells in the stimulation of primary T cell proliferation. Eur J Immunol. 1998;28:1636–44. doi: 10.1002/(SICI)1521-4141(199805)28:05<1636::AID-IMMU1636>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 33.Kelleher P, Maroof A, Knight SC. Retrovirally induced switch from production of IL-12 to IL-4 in dendritic cells. Eur J Immunol. 1999;29:2309–18. doi: 10.1002/(SICI)1521-4141(199907)29:07<2309::AID-IMMU2309>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 34.Maroof A, Penny M, Kingston R, Murray C, Islam S, Bedford PA, Knight SC. Interleukin-4 can induce interleukin-4 production in dendritic cells. Immunol. 2006;117:271–9. doi: 10.1111/j.1365-2567.2005.02305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knight SC. Lymphocyte proliferation assays. In: Klaus GGB, editor. Lymphocytes: a practical approach. Oxford: IRL Press; 1987. pp. 189–207. [Google Scholar]
- 36.Ewen C, Baca-Estrada ME. Evaluation of interleukin-4 concentration by ELISA is influenced by the consumption of IL-4 by cultured cells. J Interferon Cytokine Res. 2001;21:39–43. doi: 10.1089/107999001459141. [DOI] [PubMed] [Google Scholar]
- 37.Bedford P, Garner K, Knight SC. MHC class II molecules transferred between allogeneic dendritic cells stimulate primary mixed leukocyte reactions. Int Immunol. 1999;11:1739–44. doi: 10.1093/intimm/11.11.1739. [DOI] [PubMed] [Google Scholar]
- 38.Knight SC, Bedford PA. Dendritic cell/dendritic cell interaction. In: Thompson AW, Lotze MT, editors. Dendritic cells: biology and clinical applications. 2. London: Academic Press; 2001. pp. 333–43. [Google Scholar]
- 39.Bedford PA, Clarke LB, Hastings GZ, Knight SC. Primary proliferative responses to peptides of HIV Gag p24. J Acq Immune Defic Syndr Hum Retrovirol. 1997;14:301–6. doi: 10.1097/00042560-199704010-00001. [DOI] [PubMed] [Google Scholar]
- 40.Van Den Bogaerde JB, Kamm MA, Knight SC. Immune sensitization to food and bacteria in Crohn's disease. Gastroenterol. 1998;114:G3839. doi: 10.1046/j.1365-2036.2001.01032.x. [DOI] [PubMed] [Google Scholar]
- 41.Allan RS, Waithman J, Bedoui S, et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–62. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 42.Andre F, Chaput N, Schartz NE, et al. Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J Immunol. 2004;172:2126–36. doi: 10.4049/jimmunol.172.4.2126. [DOI] [PubMed] [Google Scholar]
- 43.Ciavarra RP, Greene AR, Horeth DR, Buhrer K, Van Rooijen N, Tedeschi B. Antigen processing of vesicular stomatitis virus in situ. Interdigitating dendritic cells present viral antigens independent of marginal dendritic cells but fail to prime CD4(+) and CD8(+) T cells. Immunology. 2000;101:512–20. doi: 10.1046/j.1365-2567.2000.t01-1-00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dolan BP, Gibbs KD, Jr, Ostrand-Rosenberg S. Tumor-specific CD4+ T cells are activated by ‘cross-dressed’ dendritic cells presenting peptide-MHC class II complexes acquired from cell-based cancer vaccines. J Immunol. 2006;176:1447–55. doi: 10.4049/jimmunol.176.3.1447. [DOI] [PubMed] [Google Scholar]
- 45.Smith AL, de St Groth BF. Antigen-pulsed CD8alpha+ dendritic cells generate an immune response after subcutaneous injection without homing to the draining lymph node. J Exp Med. 1999;189:593–8. doi: 10.1084/jem.189.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thery C, Regnault A, Garin J, et al. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999;147:599–610. doi: 10.1083/jcb.147.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knight SC, Fryer PR, Griffiths S, Harding B. Class II histocompatibility antigens on human dendritic cells. Immunol. 1987;61:21–7. [PMC free article] [PubMed] [Google Scholar]
- 48.Zitvogel L, Regnault A, Lozier A, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 49.Elvin SJ, Healey GD, Westwood A, Eyles JE, Williamson ED. Protection against heterologous B. pseudomallei strains using dendritic cell immunisation. Infect Immun. 2006;74:1706–11. doi: 10.1128/IAI.74.3.1706-1711.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Healey GD, Elvin SJ, Williamson ED. Humoral and cell-mediated adaptive immune responses are required for protection against Burkholderia pseudomallei challenge and bacterial clearance post infection. Infect Immun. 2005;73:5945–51. doi: 10.1128/IAI.73.9.5945-5951.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]