Abstract
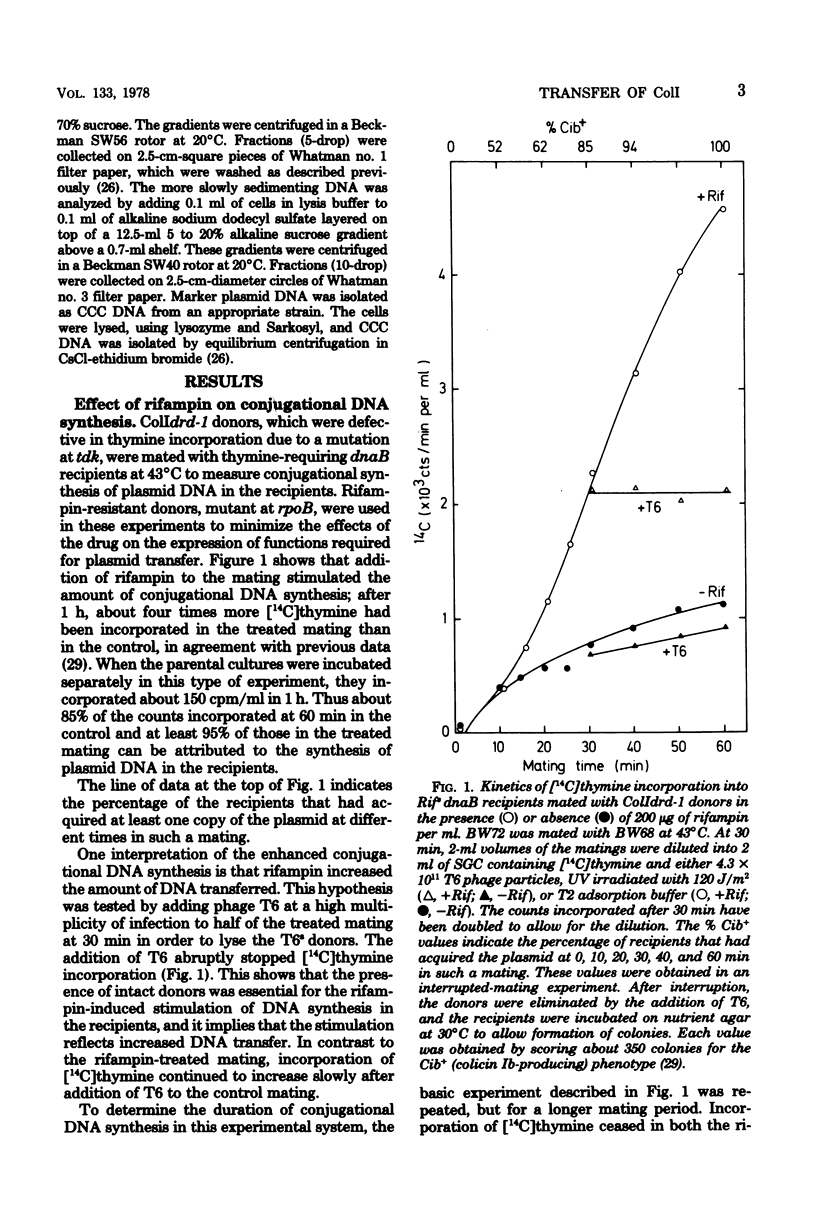
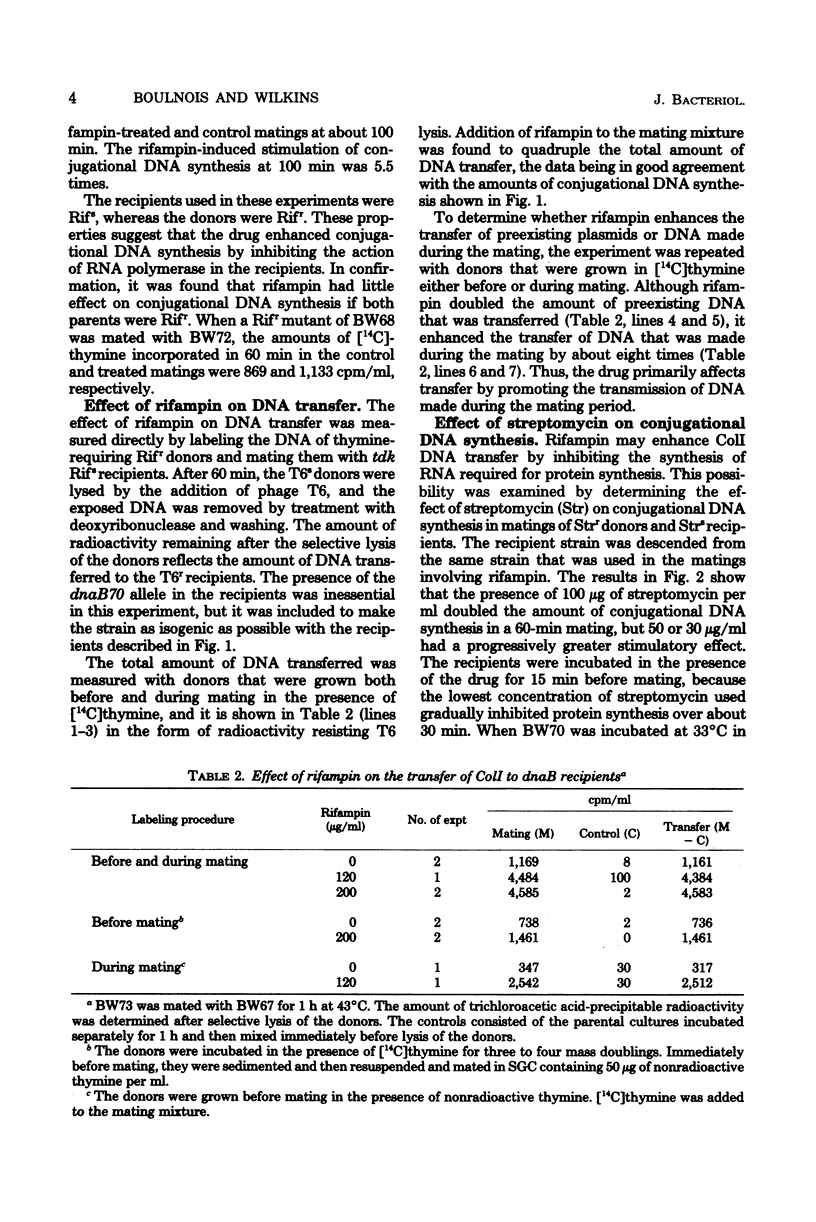
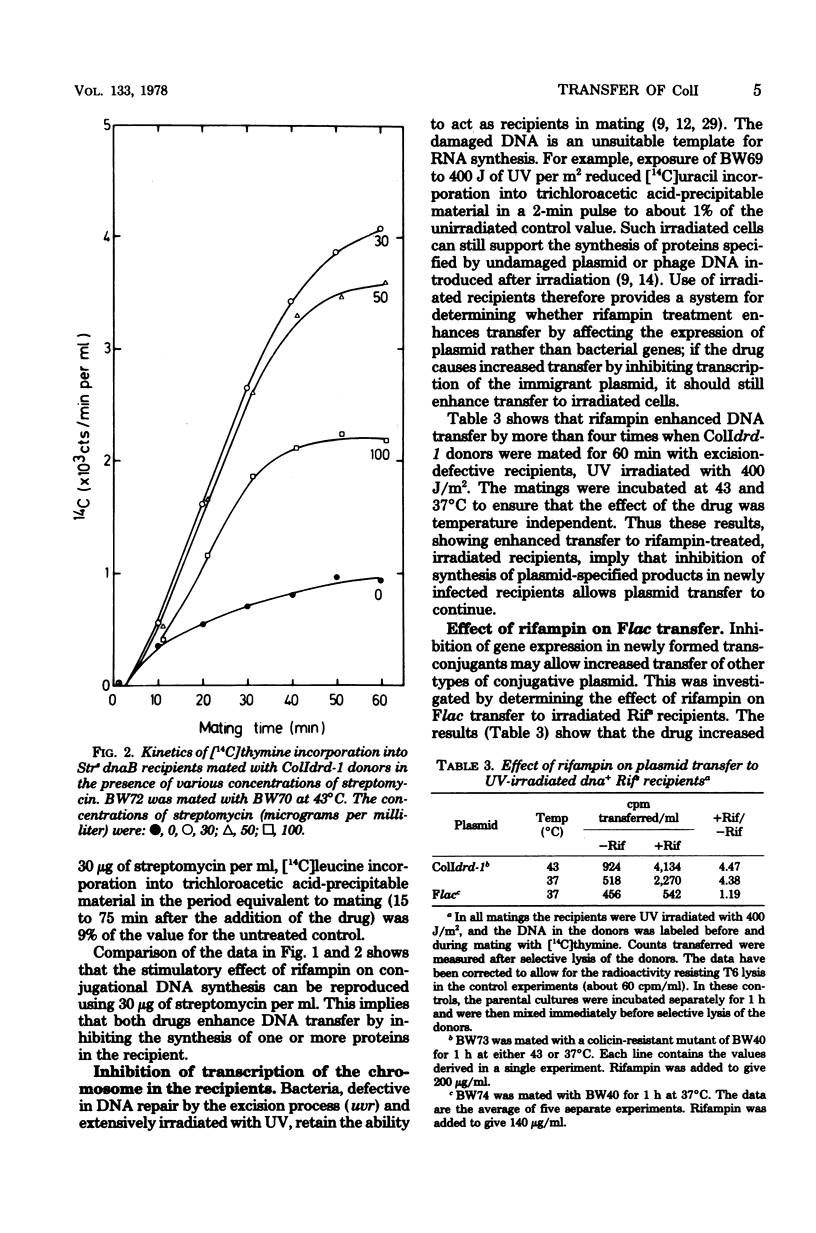
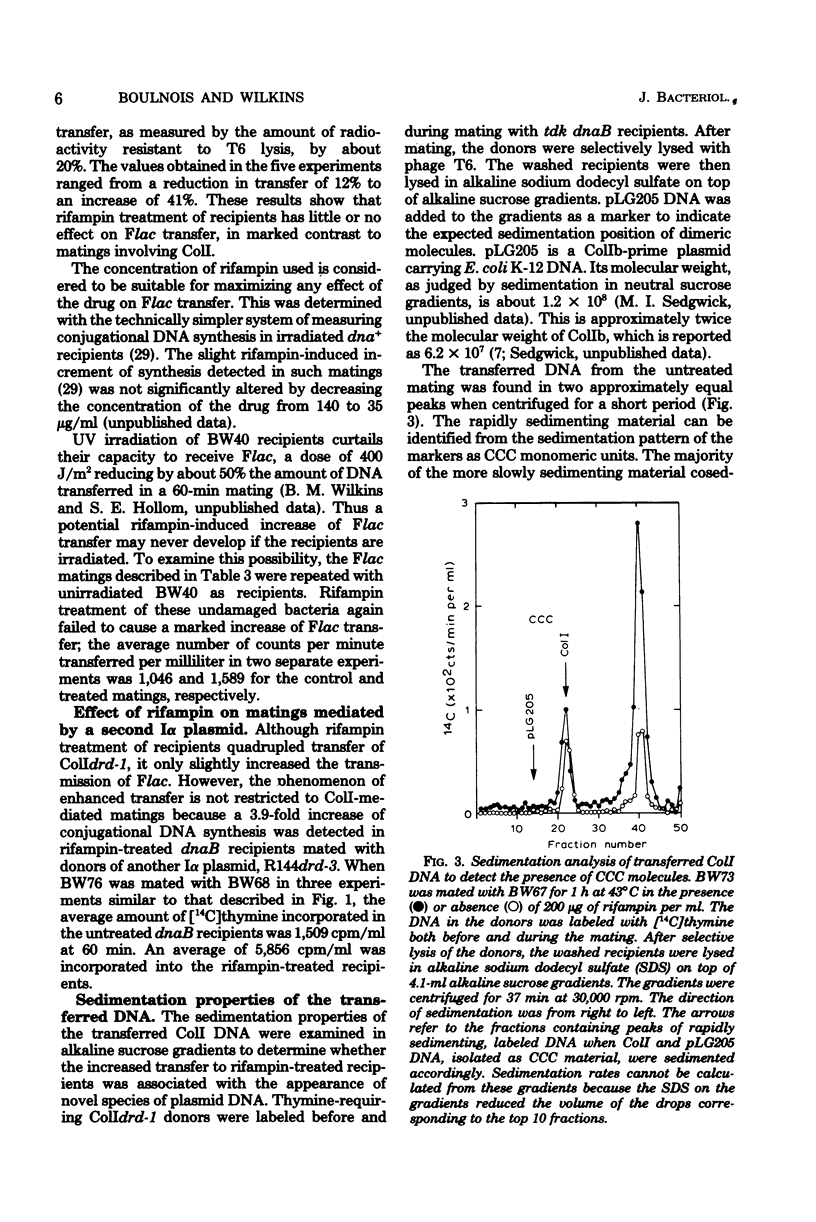
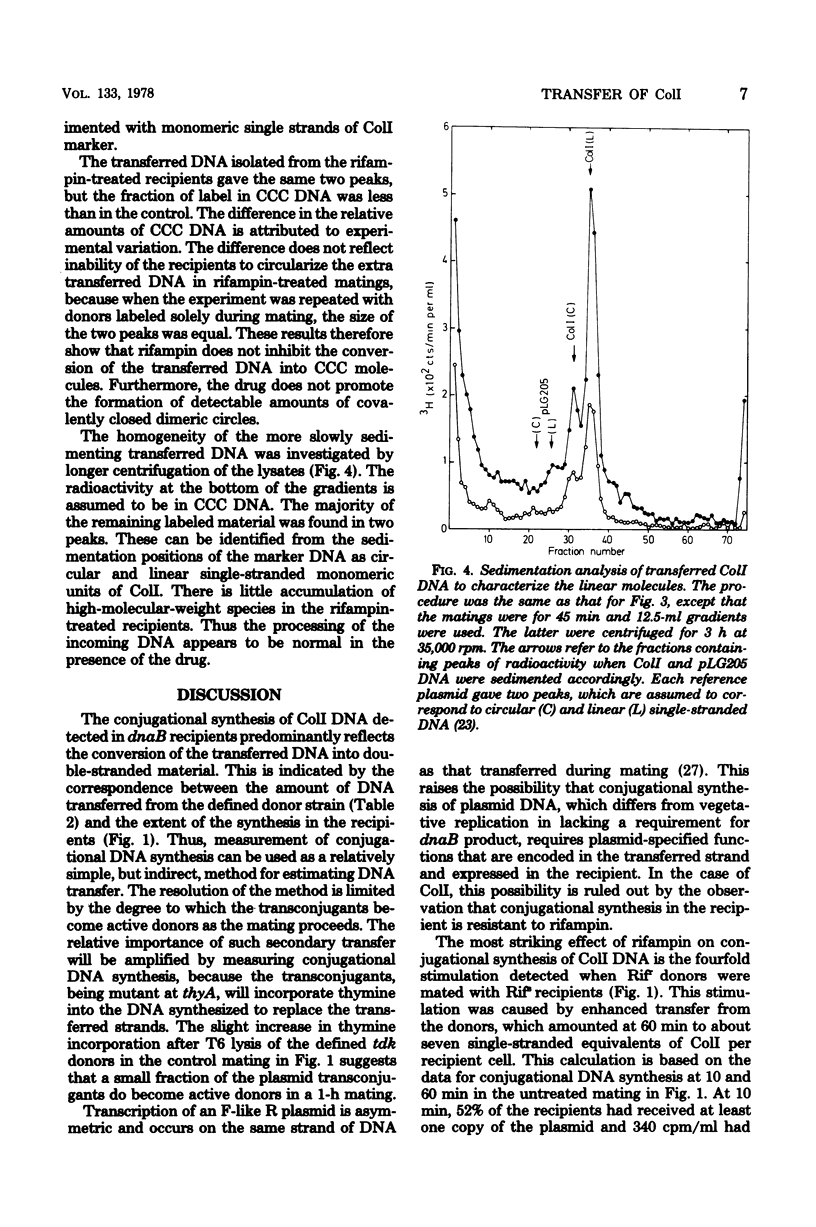
The amount of ColI DNA transferred between mating cells of Escherichia coli K-12 increased about fourfold when rifampin-resistant donors were mated with sensitive recipients in the presence of the drug. Conjugational synthesis of ColI in dnaB recipients, shown primarily to reflect conversion of the transferred DNA into double-stranded material, was also enhanced when the recipients were treated with either rifampin or streptomycin. It is suggested that the amount of ColI transfer is normally limited by the synthesis of one or more proteins in the newly infected recipients. The protein is thought to be plasmid-specified because rifampin also quadrupled transfer to UV-irradiated recipients which were deficient in the transcription of the resident DNA. Successive strands of ColI appear to be transferred discontinuously, because the transferred DNA accumulated in normal and rifampin-treated recipients in the form of circular and linear monomeric units. Although rifampin treatment of recipients also increased transfer of a second Ialpha plasmid, R144drd-3, by about four times, the drug failed to cause a substantial increase of Flac transfer in comparable matings.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANAND N., DAVIS B. D. Damage by streptomycin to the cell membrane of Escherichia coli. Nature. 1960 Jan 2;185:22–23. doi: 10.1038/185022a0. [DOI] [PubMed] [Google Scholar]
- Achtman M., Willetts N., Clark A. J. Beginning a genetic analysis of conjugational transfer determined by the F factor in Escherichia coli by isolation and characterization of transfer-deficient mutants. J Bacteriol. 1971 May;106(2):529–538. doi: 10.1128/jb.106.2.529-538.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhya S., Cleary P., Campbell A. A deletion analysis of prophage lambda and adjacent genetic regions. Proc Natl Acad Sci U S A. 1968 Nov;61(3):956–962. doi: 10.1073/pnas.61.3.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck C. F., Ingraham J. L., Neuhard J., Thomassen E. Metabolism of pyrimidines and pyrimidine nucleosides by Salmonella typhimurium. J Bacteriol. 1972 Apr;110(1):219–228. doi: 10.1128/jb.110.1.219-228.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalier-Smith T. Palindromic base sequences and replication of eukaryote chromosome ends. Nature. 1974 Aug 9;250(5466):467–470. doi: 10.1038/250467a0. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. E. Existence of the colicinogenic factor-sex factor ColI-b-P9 as a supercoiled circular DNA-protein relaxation complex. Biochem Biophys Res Commun. 1970 Oct 9;41(1):150–156. doi: 10.1016/0006-291x(70)90481-x. [DOI] [PubMed] [Google Scholar]
- Falkow S., Tompkins L. S., Silver R. P., Guerry P., Le Blanc D. J. The problems of drug-resistant pathogenic bacteria. The replication of R-factor DNA in Escherichia coli K-12 following conjugation. Ann N Y Acad Sci. 1971 Jun 11;182:153–171. doi: 10.1111/j.1749-6632.1971.tb30654.x. [DOI] [PubMed] [Google Scholar]
- Fenwick R. G., Jr, Curtiss R., 3rd Conjugal deoxyribonucleic acid replication by Escherichia coli K-12: stimulation in dnaB(ts) donors by minicells. J Bacteriol. 1973 Dec;116(3):1212–1223. doi: 10.1128/jb.116.3.1212-1223.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifelder D. R., Freifelder D. Studies on Escherichia coli sex factors. I. Specific labeling of F'Lac DNA. J Mol Biol. 1968 Feb 28;32(1):15–23. doi: 10.1016/0022-2836(68)90141-1. [DOI] [PubMed] [Google Scholar]
- Freifelder D., Folkmanis A., Kirschner I. Studies on Escherichia coli sex factors: evidence that covalent circles exist within cells and the general problem of isolation of covalent circles. J Bacteriol. 1971 Mar;105(3):722–727. doi: 10.1128/jb.105.3.722-727.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W., Dressler D. DNA replication: the rolling circle model. Cold Spring Harb Symp Quant Biol. 1968;33:473–484. doi: 10.1101/sqb.1968.033.01.055. [DOI] [PubMed] [Google Scholar]
- Hiraga S., Saitoh T. F deoxyribonucleic acid transferred to recipient cells in the presence of rifampin. J Bacteriol. 1975 Mar;121(3):1000–1006. doi: 10.1128/jb.121.3.1000-1006.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOW B., WOOD T. H. A QUICK AND EFFICIENT METHOD FOR INTERRUPTION OF BACTERIAL CONJUGATION. Genet Res. 1965 Jul;6:300–303. doi: 10.1017/s001667230000416x. [DOI] [PubMed] [Google Scholar]
- Matsubara K. Properties of sex factor and related episomes isolated from purified Escherichia coli zygote cells. J Mol Biol. 1968 Nov 28;38(1):89–108. doi: 10.1016/0022-2836(68)90130-7. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Clowes R. C., Cohen S. N., Curtiss R., 3rd, Datta N., Falkow S. Uniform nomenclature for bacterial plasmids: a proposal. Bacteriol Rev. 1976 Mar;40(1):168–189. doi: 10.1128/br.40.1.168-189.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P. Extrachromosomal inheritance in bacteria. Bacteriol Rev. 1969 Jun;33(2):210–263. doi: 10.1128/br.33.2.210-263.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades M., Thomas C. A., Jr The P22 bacteriophage DNA molecule. II. Circular intracellular forms. J Mol Biol. 1968 Oct 14;37(1):41–61. doi: 10.1016/0022-2836(68)90072-7. [DOI] [PubMed] [Google Scholar]
- Rupp W. D., Ihler G. Strand selection during bacterial mating. Cold Spring Harb Symp Quant Biol. 1968;33:647–650. doi: 10.1101/sqb.1968.033.01.073. [DOI] [PubMed] [Google Scholar]
- Vapnek D., Lipman M. B., Rupp W. D. Physical properties and mechanism of transfer of R factors in Escherichia coli. J Bacteriol. 1971 Oct;108(1):508–514. doi: 10.1128/jb.108.1.508-514.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vapnek D., Rupp W. D. Asymmetric segregation of the complementary sex-factor DNA strands during conjugation in Escherichia coli. J Mol Biol. 1970 Nov 14;53(3):287–303. doi: 10.1016/0022-2836(70)90066-5. [DOI] [PubMed] [Google Scholar]
- Vapnek D., Spingler E. Asymmetry and extent of in vivo transcripition of R-plasmid deoxyribonucleic acid in Escherichia coli. J Bacteriol. 1974 Dec;120(3):1274–1278. doi: 10.1128/jb.120.3.1274-1278.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins B. M., Hollom S. E. Conjugational synthesis of F lac+ and Col I DNA in the presence of rifampicin and in Escherichia coli K12 mutants defective in DNA synthesis. Mol Gen Genet. 1974;134(2):143–156. doi: 10.1007/BF00268416. [DOI] [PubMed] [Google Scholar]
- Wilkins B. M., Hollom S. E., Rupp W. D. Deoxyribonucleic acid transferred from ultraviolet-irradiated excision-defective Hfr cells of Escherichia coli K-12. J Bacteriol. 1971 Aug;107(2):505–512. doi: 10.1128/jb.107.2.505-512.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins B. M. Partial suppression of the phenotype of Escherichia coli K-12 dnaG mutants by some I-like conjugative plasmids. J Bacteriol. 1975 Jun;122(3):899–904. doi: 10.1128/jb.122.3.899-904.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaritsky A., Pritchard R. H. Replication time of the chromosome in thymineless mutants of Escherichia coli. J Mol Biol. 1971 Aug 28;60(1):65–74. doi: 10.1016/0022-2836(71)90447-5. [DOI] [PubMed] [Google Scholar]


