Abstract
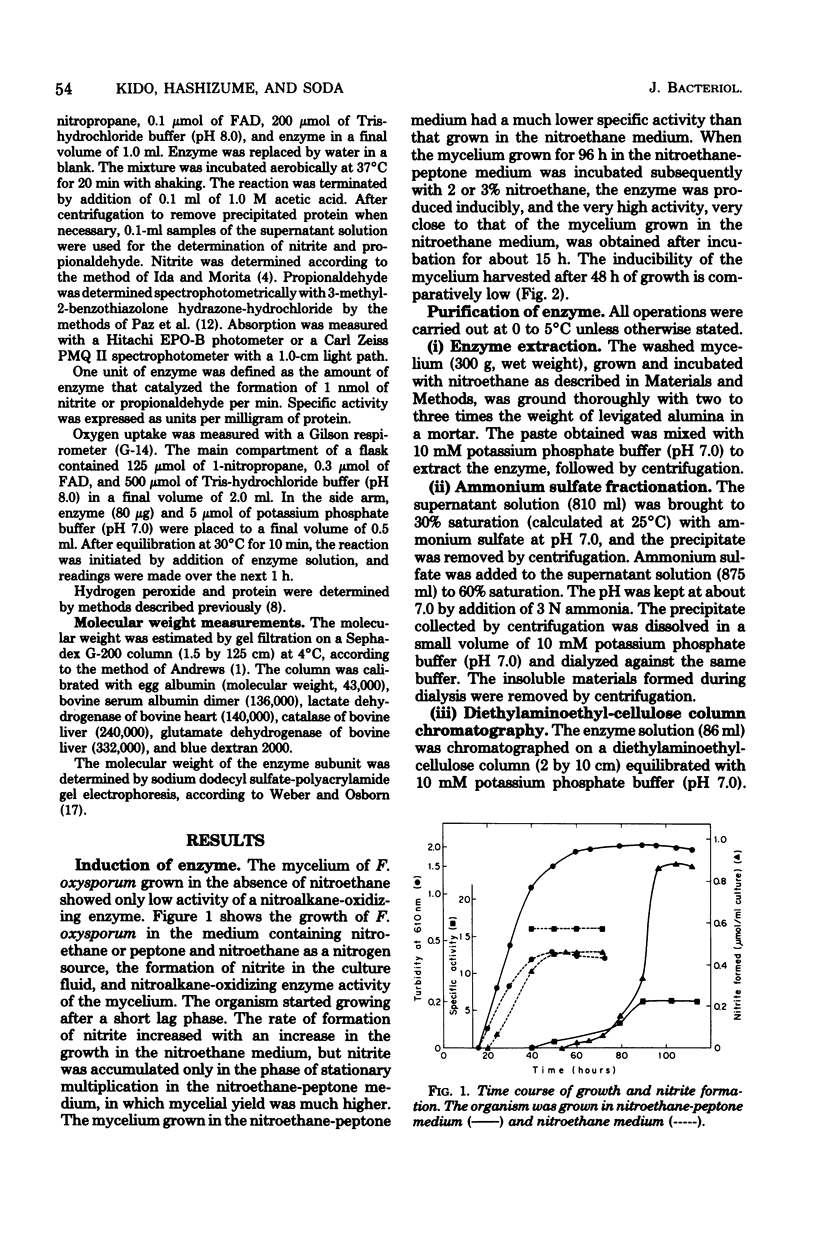
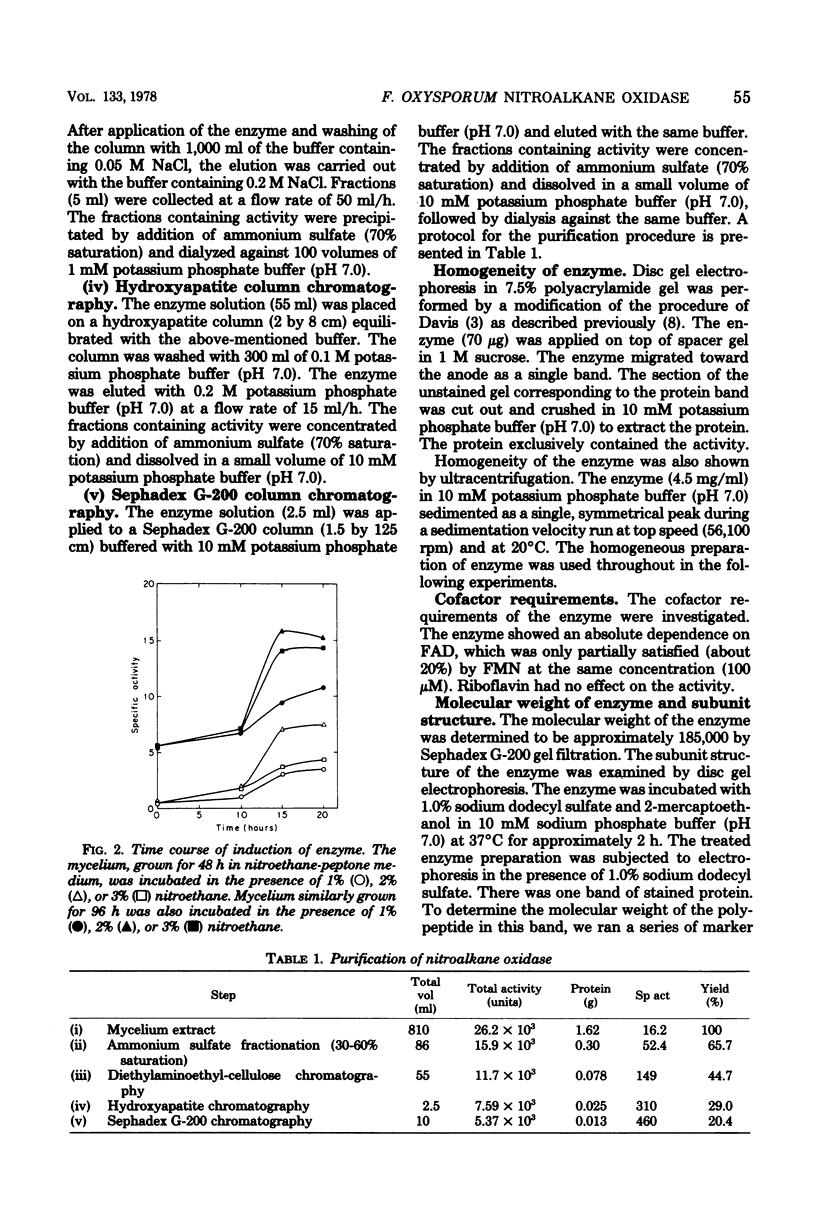
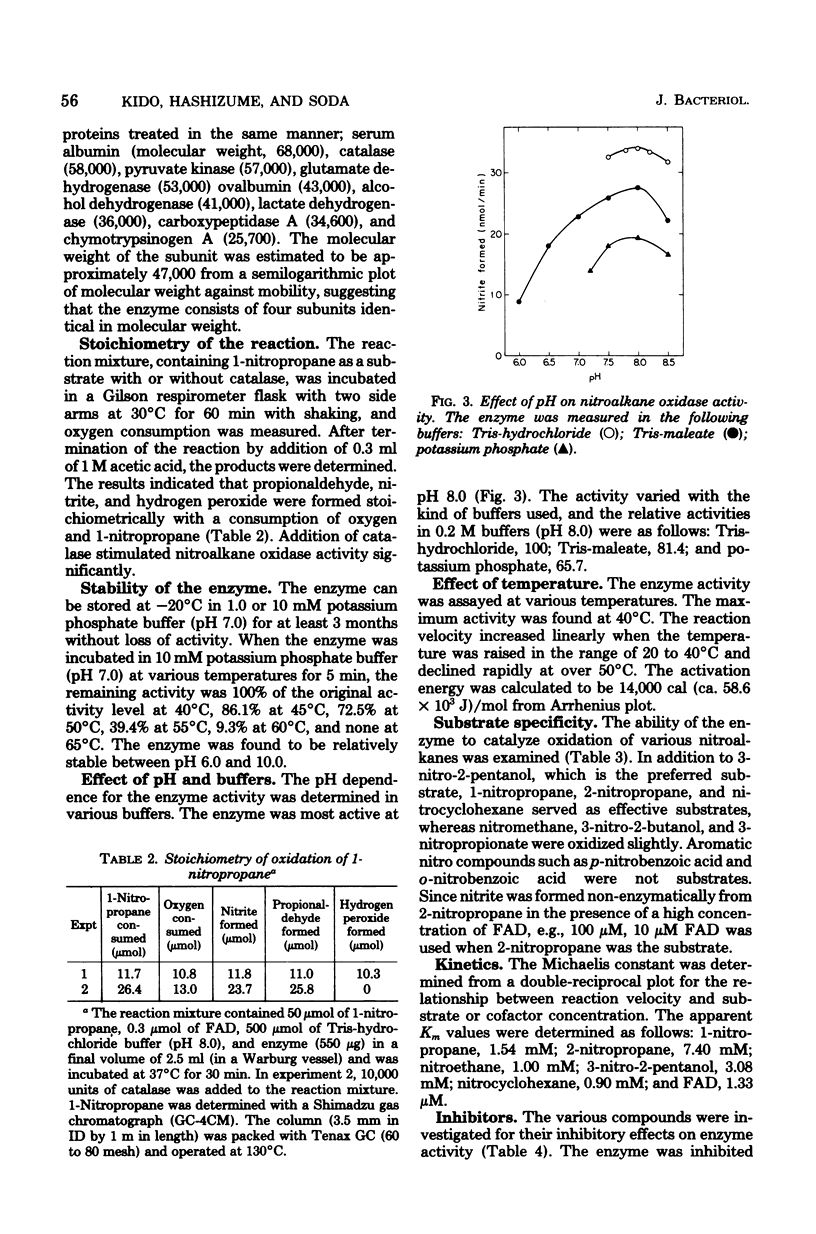
A nitroalkane-oxidizing enzyme, which was inducibly formed by addition of nitroethane to the medium was purified to homogeneity from an extract of Fusarium oxysporum (IFO 5942) with an overall yield of about 20%. The enzyme catalyzed the oxidative denitrification of 1-nitropropane as follows: CH2(NO2)CH2CH3 + O2 + H2O leads to OHCCH2CH3 + HNO2 + H2O2. In addition to 1-nitropropane, 3-nitro-2-pentanol, 2-nitropropane, and nitrocyclohexane are good substrates; the enzyme is designated "nitroalkane oxidase" (EC class 1.7.3). The enzyme has a molecular weight of approximately 185,000 and consists of four subunits identical in molecular weight (47,000). Flavin adenine dinucleotide was required for the enzyme activity and could be replaced in part by riboflavin 5'-phosphate. The maximum reactivity was found at about pH 8.0. The enzyme was inhibited significantly by HgCl2, KCN, p-chloromercuribenzoate, and N-ethylmaleimide. The Michaelis constants are as follows: 1-nitropropane, 1.54 mM; 2-nitropropane, 7.40 mM; nitroethane, 1.00 mM; 3-nitro-2-pentanol, 3.08 mM; nitrocyclohexane, 0.90 mM; and flavin adenine dinucleotide, 1.33 micrometer.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Kido T., Soda K., Suzuki T., Asada K. A new oxygenase, 2-nitropropane dioxygenase of Hansenula mrakii. Enzymologic and spectrophotometric properties. J Biol Chem. 1976 Nov 25;251(22):6994–7000. [PubMed] [Google Scholar]
- Kido T., Yamamoto T., Soda K. Microbial assimilation of alkyl nitro compounds and formation of nitrite. Arch Microbiol. 1975 Dec 31;106(3):165–169. doi: 10.1007/BF00446519. [DOI] [PubMed] [Google Scholar]
- Kido T., Yamamoto T., Soda K. Purification and properties of nitroalkane-oxidizing enzyme from Hansenula mrakii. J Bacteriol. 1976 Jun;126(3):1261–1265. doi: 10.1128/jb.126.3.1261-1265.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITTLE H. N. Oxidation of nitroethane by extracts from Neurospora. J Biol Chem. 1951 Nov;193(1):347–358. [PubMed] [Google Scholar]
- LITTLE H. N. The oxidation of 2-nitropropane by extracts of pea plants. J Biol Chem. 1957 Nov;229(1):231–238. [PubMed] [Google Scholar]
- PAZ M. A., BLUMENFELD O. O., ROJKIND M., HENSON E., FURFINE C., GALLOP P. M. DETERMINATION OF CARBONYL COMPOUNDS WITH N-METHYL BENZOTHIAZOLONE HYDRAZONE. Arch Biochem Biophys. 1965 Mar;109:548–559. doi: 10.1016/0003-9861(65)90400-5. [DOI] [PubMed] [Google Scholar]
- Takeda H., Yamamoto S., Kojima Y., Hayaishi O. Studies on monooxygenases. I. General properties of crystalline L-lysine monooxygenase. J Biol Chem. 1969 Jun 10;244(11):2935–2941. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


