Abstract
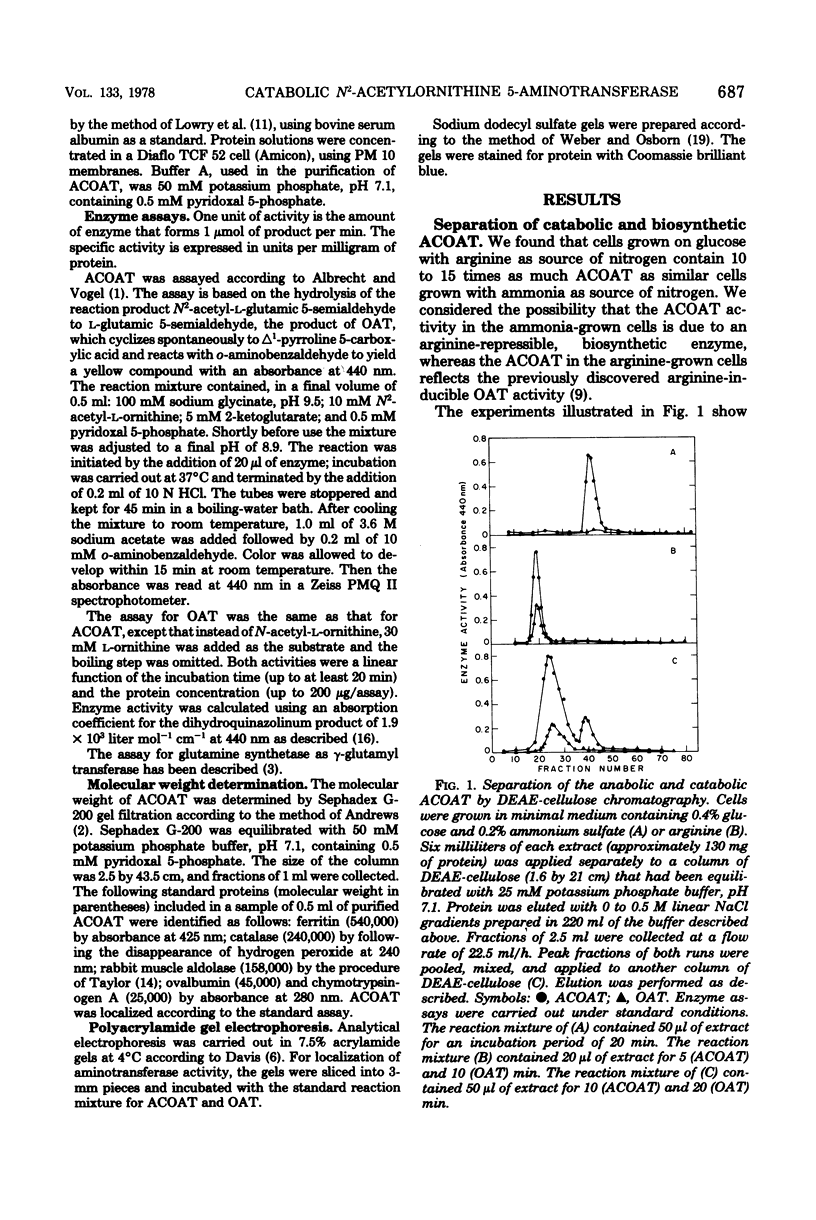
Klebsiella aerogenes formed two N2-acetylornithine 5-aminotransferases (ACOAT) which were separable by diethylaminoethyl-cellulose chromatography. One ACOAT was repressed when the cells grew on arginine-containing medium, indicating its function in arginine biosynthesis. The second ACOAT was induced when arginine or ornithine was present in the medium as the sole source of carbon or nitrogen, suggesting its function in the catabolism of these compounds. The induced enzyme was purified almost to homogeneity. Its molecular weight is 59,000; it is a pyridoxal 5-phosphate-dependent enzyme and exhibits activity with N2-acetylornithine (Km = 1.1 mM) as well as with ornithine (Km = 5.4 mM). ACOAT did not catalyze the transamination of putrescine or 4-aminobutyrate. The best amino acceptor was 2-ketoglutarate (Km = 0.7 mM). ACOAT formation was subject to catabolite repression exerted by glucose when ammonia was present in excess. When the cells were deprived of nitrogen, ACOAT escaped from catabolite repression. This activation was mediated by glutamine synthetase as shown by the fact that mutants affected in the regulation or synthesis of glutamine synthetase were also affected in the control of ACOAT formation.
Full text
PDF
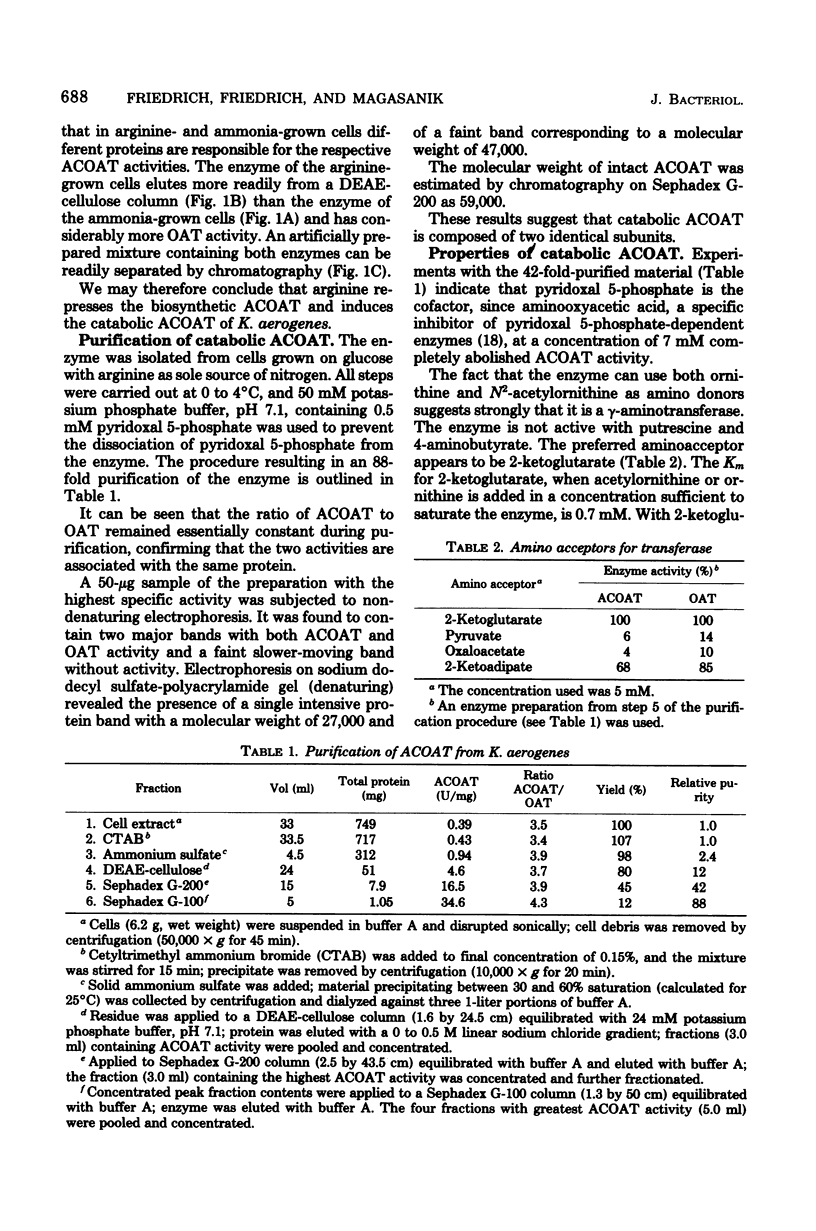



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBRECHT A. M., VOGEL H. J. ACETYLORNITHINE DELTA-TRANSAMINASE. PARTIAL PURIFICATION AND REPRESSION BEHAVIOR. J Biol Chem. 1964 Jun;239:1872–1876. [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender R. A., Janssen K. A., Resnick A. D., Blumenberg M., Foor F., Magasanik B. Biochemical parameters of glutamine synthetase from Klebsiella aerogenes. J Bacteriol. 1977 Feb;129(2):1001–1009. doi: 10.1128/jb.129.2.1001-1009.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billheimer J. T., Carnevale H. N., Leisinger T., Eckhardt T., Jones E. E. Ornithine delta-transaminase activity in Escherichia coli: its identity with acetylornithine delta-transaminase. J Bacteriol. 1976 Sep;127(3):1315–1323. doi: 10.1128/jb.127.3.1315-1323.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billheimer J. T., Jones E. E. Inducible and repressible acetylornithine delta-transaminase in Escherichia coli: different proteins. Arch Biochem Biophys. 1974 Apr 2;161(2):647–651. doi: 10.1016/0003-9861(74)90349-x. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DE DEKEN R. H. Pathway of arginine biosynthesis in yeast. Biochem Biophys Res Commun. 1962 Aug 31;8:462–466. doi: 10.1016/0006-291x(62)90297-8. [DOI] [PubMed] [Google Scholar]
- Friedrich B., Magasanik B. Urease of Klebsiella aerogenes: control of its synthesis by glutamine synthetase. J Bacteriol. 1977 Aug;131(2):446–452. doi: 10.1128/jb.131.2.446-452.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich B., Magasanik B. Utilization of arginine by Klebsiella aerogenes. J Bacteriol. 1978 Feb;133(2):680–685. doi: 10.1128/jb.133.2.680-685.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac J. H., Holloway B. W. Control of arginine biosynthesis in Pseudomonas aeruginosa. J Gen Microbiol. 1972 Dec;73(3):427–438. doi: 10.1099/00221287-73-3-427. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Prival M. J., Brenchley J. E., Magasanik B. Glutamine synthetase and the regulation of histidase formation in Klebsiella aerogenes. J Biol Chem. 1973 Jun 25;248(12):4334–4344. [PubMed] [Google Scholar]
- Prival M. J., Magasanik B. Resistance to catabolite repression of histidase and proline oxidase during nitrogen-limited growth of Klebsiella aerogenes. J Biol Chem. 1971 Oct 25;246(20):6288–6296. [PubMed] [Google Scholar]
- Tyler B., Deleo A. B., Magasanik B. Activation of transcription of hut DNA by glutamine synthetase. Proc Natl Acad Sci U S A. 1974 Jan;71(1):225–229. doi: 10.1073/pnas.71.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voellmy R., Leisinger T. Dual role for N-2-acetylornithine 5-aminotransferase from Pseudomonas aeruginosa in arginine biosynthesis and arginine catabolism. J Bacteriol. 1975 Jun;122(3):799–809. doi: 10.1128/jb.122.3.799-809.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLACH D. P. Studies on the GABA pathway. I. The inhibition of gamma-aminobutyric acid-alpha-ketoglutaric acid transaminase in vitro and in vivo by U-7524 (amino-oxyacetic acid). Biochem Pharmacol. 1961 Feb;5:323–331. doi: 10.1016/0006-2952(61)90023-5. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


