Abstract
Regulation of vitamin D metabolism has long been examined by using vitamin D-deficient hypocalcemic animals. We previously reported that, in a rat model of chronic hyperparathyroidism, expression of 25-hydroxyvitamin D3-1α-hydroxylase (CYP27B1) mRNA was markedly increased in renal proximal convoluted tubules. It is believed that the major regulator for the expression of renal CYP27B1 is parathyroid hormone (PTH). However, in the normocalcemic state, the mechanism to regulate the renal CYP27B1 gene could be different, since plasma levels of PTH are very low. In the present study, the effect of PTH and calcitonin (CT) on the expression of renal CYP27B1 mRNA was investigated in normocalcemic sham-operated rats and normocalcemic thyroparathyroidectomized (TPTX) rats generated by either PTH or CaCl2 infusion. A single injection of CT dose-dependently decreased the expression of vitamin D receptor mRNA in the kidney of normocalcemic sham-TPTX rats. Concomitantly, CT greatly increased the expression of CYP27B1 mRNA in the kidney of normocalcemic sham-TPTX rats. CT also increased the expression of CYP27B1 mRNA in the kidney of normocalcemic TPTX rats. Conversion of serum [3H]1α,25(OH)2D3 from 25-hydroxy[3H]vitamin D3 in vivo was also greatly increased by the injection of CT into sham-TPTX rats and normocalcemic TPTX rats, but not into hypocalcemic TPTX rats. In contrast, administration of PTH did not induce the expression of CYP27B1 mRNA in the kidney of vitamin D-replete sham-TPTX rats and hypocalcemic TPTX rats. PTH increased the expression of renal CYP27B1 mRNA only in vitamin D-deficient hypocalcemic TPTX rats. These results suggest that CT plays an important role in the maintenance of serum 1α,25(OH)2D3 under normocalcemic physiological conditions, at least in rats.
Keywords: vitamin D metabolism, 1α-hydroxylase, parathyroid hormone
It is well established that vitamin D3 is first metabolized in the liver to 25-hydroxyvitamin D3 [25(OH)D3], then in the kidney to 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3] and 24,25-dihydroxyvitamin D3 [24,25(OH)2D3]. Of these two dihydroxyvitamin D3 metabolites, 1α,25(OH)2D3 has been shown to be the active form of vitamin D3 (1, 2). Vitamin D metabolism has been investigated mainly in terms of enzyme activity. With regard to the enzyme activity, the renal metabolism of 25(OH)D3 is tightly regulated by serum levels of various ions and hormones (1–3). Among these, parathyroid hormone (PTH) is a major inducer of 25(OH)D3-1α-hydroxylase (CYP27B1) activity (3, 4). 1α,25(OH)2D3 is the only compound that has been shown to induce renal 25(OH)D3-24-hydroxylase (CYP24) (1–3). Furthermore, renal CYP27B1 activity was strongly inhibited by the administration of 1α,25(OH)2D3. Thus, it is suggested that the renal CYP27B1 activity is regulated mainly by a balance of the serum levels of 1α,25(OH)2D3 and PTH.
Another calcium-regulating hormone, calcitonin (CT), was also shown to enhance renal conversion of 25(OH)D3 into 1α,25(OH)2D3 in vitamin D-deficient rats (5–7). Lorenc et al. (6), however, showed that CT did not exhibit any effect on vitamin D metabolism, when animals were thyroparathyroidectomized (TPTX). From these results, they concluded that the stimulatory effect of CT was mediated by the endogenous secretion of PTH. The effect of CT on the 1α,25(OH)2D3 synthesis and its interaction with PTH are of considerable interest, since the stimulatory effect of PTH on 1α,25(OH)2D3 synthesis appears to be mediated by cAMP (4), and CT also stimulates cAMP production in the kidney, irrespective of the counteracting effect of CT on PTH in maintaining plasma calcium homeostasis (8).
It is believed that 1α,25(OH)2D3 is responsible for inducing most of the biological functions of vitamin D3 (1–3). Theoretically, derangements in the tissue and plasma levels of 1α,25(OH)2D3 could result from alterations in the rate of its synthesis or degradation or both. Recently, four laboratories independently succeeded in the molecular cloning of the cDNA for CYP27B1 (9–13). The cloning of the CYP27B1 cDNA has enabled us to examine the expression of mRNA of this enzyme. Using this probe, we found that the expression of CYP27B1 mRNA was greatly increased in the kidney of vitamin D-deficient rats. In rats with the enhanced production of 1α,25(OH)2D3, expression of CYP27B1 mRNA was also greatly stimulated in renal proximal convoluted tubules (9). Brenza et al. (14) and Murayama et al. (15) also reported that a PTH-dependent positive regulatory element for the CYP27B1 gene expression is located in the promoter region of the gene.
Regulation of vitamin D metabolism has long been examined by using hypocalcemic or hypophosphatemic vitamin D-deficient animals. It was difficult to examine the regulation of CYP27B1 activity in normocalcemic physiological conditions. 1α,25(OH)2D3 has been characterized as a negative regulator for the activity of renal CYP27B1 in hypocalcemic or hypophosphatemic conditions (1–3). However, in the normocalcemic state, the mechanism to maintain the serum levels of 1α,25(OH)2D3 could be different, since plasma levels of PTH are very low. The aim of the present study is to clarify the mechanism and regulation of CYP27B1 gene expression under a normocalcemic physiological condition. We report here that CT, but not PTH, is a major stimulator of the expression of CYP27B1 mRNA in the kidney of normocalcemic rats.
MATERIALS AND METHODS
Animals.
Male weanling rats (Sprague–Dawley strain) were maintained on a synthetic vitamin D-deficient diet containing either 0.03% Ca (low Ca) or 0.5% Ca (adequate Ca) (Teklad, Madison, WI). All diets contained 0.6% phosphorus. Rats fed either vitamin D-deficient diet were maintained in a room with incandescent lighting, and all potential sources of ultraviolet light and vitamin D were excluded. Thyroparathyroidectomy (TPTX) was performed under light ether anesthesia and the glandular tissues were removed by blunt dissection. The validity of the procedure was assessed by measuring the levels of serum Ca on the second day after the surgery. All TPTX rats were administered 4 μg of l-thyroxin (Sigma) every other day. Rat PTH-(1–34) was purchased from Peninsula Laboratories. Eel CT and cDNA for rat CT receptor (CTR) were kindly donated by Asahi Chemicals (Shizuoka, Japan). 1α,25(OH)2D3 was purchased from Wako Pure Chemicals (Osaka, Japan). Adenosine 3′,5′-cyclic monophosphate monosodium salt (cAMP) was obtained from Sigma. [26,27-3H]25(OH)D3 (specific activity, 1.03 TBq/mmol) was obtained from Amersham International, Bucks, U.K. [α-32P]dCTP (specific activity, 111 TBq/mmol) was purchased from Du Pont-New England Nuclear. All chemicals were of a highly purified analytical grade.
Northern Blot Analysis of CYP27B1 mRNA.
Total RNA was prepared by the method of Chomczynski and Sacchi (16), and poly(A)+ RNA was isolated with the Oligotex dT 30 isolation system (Takara Biomedicals, Shiga, Japan). Samples (5 μg) of poly(A)+ RNA were denatured by heating at 70°C in 20 mM Mops, pH 7.0, containing 5 mM sodium acetate, 1 mM EDTA, 2.2 M formaldehyde, and 50% formamide for 5 min and subjected to electrophoresis in 1.2% agarose gels containing 2.2 M formaldehyde. Resolved RNA was transferred to a Hybond-N membrane (Amersham Pharmacia Biotech) and covalently crosslinked by exposure to UV light. RNA blots were prehybridized for 4 h at 42°C in a mixture containing 50% formamide, 5× SSC, 5 μg/ml denatured salmon sperm DNA, and 1× Denhardt’s solution. cDNA probes used in this experiment were as follows: CYP27B1; the 1.5-kb EcoRI and BamHI fragment of rat 1α-hydroxylase cDNA; vitamin D receptor (VDR), the 1.8-kb EcoRI fragment of rat intestinal 1α,25(OH)2D3 receptor cDNA, CTR; the 2.2-kb HindIII fragment of rat CTR cDNA; and β-actin, the 0.4-kb HinfI fragment of human β-actin cDNA. All cDNAs were labeled with [α-32P]dCTP by the random priming procedure of Feinberg et al. (17). The membranes were exposed to Kodak XAR films at −80°C with an intensifying screen.
Analysis of in Vivo Metabolism of 25(OH)D3.
At 6 h after PTH or CT injection, 20 kBq of [3H]25(OH)D3 was injected intravenously into 5-week-old sham-operated or TPTX rats. The rats were killed 6 h later and their blood was collected. The blood plasma was extracted with methanol and chloroform by the method of Bligh and Dyer (18). An aliquot of the lipid-soluble metabolites was subjected to HPLC under the following conditions: column, Finepak SIL 4.6 × 250 mm; mobile phase, n-hexane/2-propanol/methanol (89:5.5:5.5, vol/vol); flow rate, 1.0 ml/min (19). The radioactivity of the eluate was measured. The serum level of 25(OH)D3 was determined by the competitive protein binding assay (CPBA), using serum obtained from rachitic rats as binding protein.
RESULTS
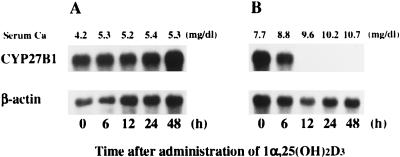
Fig. 1 shows the time course of change in the expression of renal CYP27B1 mRNA and in the increase of serum calcium after a single injection of 1α,25(OH)2D3 into vitamin D-deficient rats fed a low (0.03%) Ca or an adequate (0.5%) Ca diet. A significant increase in the serum Ca was observed 6 h after the administration of 1α,25(OH)2D3 into rats fed a vitamin D-deficient diet containing 0.5% Ca (Fig. 1B). At 12 h, serum Ca levels attained a normocalcemic range. In contrast, in the low-Ca (0.03%) group, the serum level of Ca was increased, but only slightly (from 4.2 to 5.3 mg/dl) even at 48 h after 1α,25(OH)2D3 injection (Fig. 1A). In vitamin D-deficient rats fed an adequate Ca diet, expression of CYP27B1 mRNA decreased as early as 6 h after 1α,25(OH)2D3 injection, and it disappeared completely at 12 h (Fig. 1B). In contrast, administration of 1α,25(OH)2D3 did not at all influence the expression of CYP27B1 mRNA in the kidney of vitamin D-deficient rats fed the low-Ca diet (Fig. 1A). Administration of 1α,25(OH)2D3 did not affect significantly the renal expression of β-actin in rats fed either the adequate or the low-Ca diet.
Figure 1.
Effect of dietary calcium on the suppression of renal CYP27B1 mRNA expression by 1α,25(OH)2D3. Male weanling rats were maintained for 2 weeks on a synthetic vitamin D-deficient, low-Ca diet (0.03% Ca, 0.6% P), then for a week on a vitamin D-deficient diet containing either 0.03% (A) or 0.5% (B) Ca. At the end of the feeding period, 1α,25(OH)2D3 (1 μg per rat) was injected intravenously. Renal poly(A)+ RNA was purified from total RNA by using Oligotex dT 30, and Northern blots were probed with cDNAs for CYP27B1 and β-actin. Similar results were obtained in three independent sets of experiments.
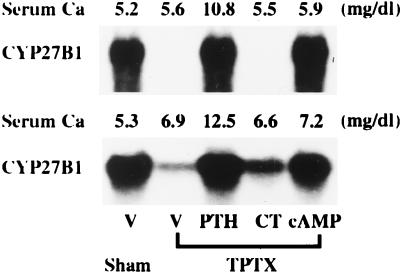
We next examined the effects of PTH, CT, and cAMP on the expression of CYP27B1 mRNA in vitamin D-deficient TPTX rats after continuous infusion of each hormone or cyclic nucleotide. After TPTX, vitamin D-deficient rats could not survive without Ca infusion. Infusion of 5 mM CaCl2 into vitamin D-deficient TPTX rats (Fig. 2 Upper) maintained the serum Ca concentration within the level in vitamin D-deficient rats. A continuous infusion of 10 mM CaCl2 into vitamin D-deficient TPTX rats (Fig. 2 Lower) increased the serum Ca concentration from 5.3 to 6.9 mg/dl at 48 h. Infusion of PTH or cAMP markedly increased the renal expression of CYP27B1 mRNA in vitamin D-deficient TPTX rats concomitantly infused with either 5 mM (Fig. 2 Upper) or 10 mM (Fig. 2 Lower) CaCl2. Administration of CT induced a slight but significant increase in the expression of renal CYP27B1 mRNA in TPTX rats infused with 10 mM CaCl2 (Fig. 2 Lower) but not with 5 mM CaCl2 (Fig. 2 Upper).
Figure 2.
Comparative effects of CT, PTH, and cAMP on the expression of renal CYP27B1 mRNA in vitamin D-deficient TPTX rats infused with 5 mM or 10 mM CaCl2. Male weanling rats were maintained for 3 weeks on a synthetic vitamin D-deficient, low-Ca diet (0.03% Ca, 0.6% P). At the end of the feeding period, rats were TPTX under light ether anesthesia and were infused with either 5 mM (Upper) or 10 mM (Lower) CaCl2 at a rate of 3 ml/h for 48 h. Infusion of either 5 mM or 10 mM CaCl2 for 48 h increased the serum levels of Ca to 5.6 and 6.9 mg/dl, respectively. CT, PTH, cAMP, or vehicle (V: sodium acetate) was administered for the last 12 h of the infusion period at a dose of 0.5 unit/h, 3 μg/h, 250 nmol/h, or 0.5 nmol/h, respectively. Renal poly(A)+ RNA was purified from total RNA by using Oligotex dT 30, and Northern blots were probed with cDNAs for CYP27B1. Similar results were obtained in three independent sets of experiments.
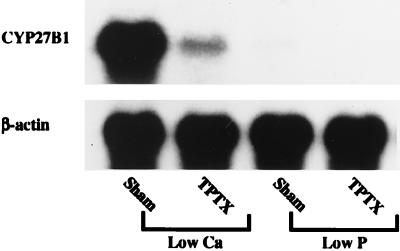
Previously we reported that the expression of CYP27B1 mRNA was increased significantly in the proximal convoluted tubules of rats fed a low-Ca diet, whose kidneys showed enhanced 1α,25(OH)2D3 production (20). When weanling rats were sham-operated or TPTX and then fed a vitamin D-replete, low-Ca diet for 8 days, TPTX strikingly decreased the renal expression of CYP27B1 mRNA (Fig. 3). Low-phosphorus (0.03%) feeding of sham-TPTX rats for 8 days had little effect on the renal expression of CYP27B1 mRNA. This result indicates that the increase in the level of CYP27B1 mRNA in sham-TPTX rats fed a low-Ca diet is primarily because of the enhanced secretion of PTH. In contrast to the low-Ca feeding, continuous infusion of PTH (3 μg/h for 3 days) into sham-TPTX rats did not induce the renal expression of CYP27B1 mRNA (data not shown).
Figure 3.
Effects of low-calcium and low-phosphorus feedings on the expression of CYP27B1 mRNA in the kidney of TPTX rats. Male weanling rats fed a laboratory chow containing 2 units of vitamin D3 per gram of diet were either sham-operated or TPTX under light ether anesthesia. After the surgery, rats were fed a vitamin D-replete, low-calcium (0.03% Ca, 0.6% P) or low-phosphorus diet (0.6% Ca, 0.02% P) for 8 days. Renal poly(A)+ RNA was purified from total RNA by using Oligotex dT 30, and Northern blots were probed with cDNAs for CYP27B1 and β-actin. Similar results were obtained in four independent sets of experiments.
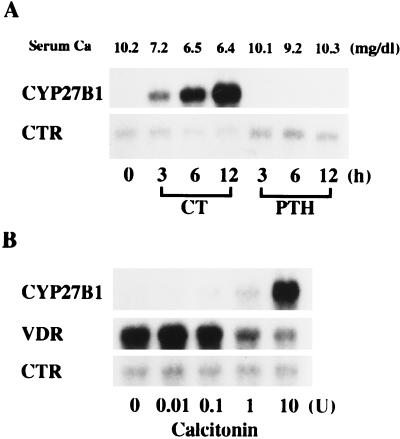
Fig. 4 shows the time- and dose-dependent expression of CYP27B1, VDR, and CTR mRNAs in the kidney of sham-TPTX rats after a single injection of CT or PTH. A single injection of PTH had no effect on the serum level of Ca in sham-TPTX rats. In contrast, CT decreased the serum level of Ca in a time-dependent manner. The control level of CYP27B1 mRNA in the kidney of sham-TPTX rats was almost undetectable. A single injection of CT into sham-TPTX rats increased the expression of CYP27B1 mRNA in a time- and dose-dependent manner (Fig. 4 A and B). Concomitantly, the expression of VDR mRNA was down-regulated by CT in a dose-dependent manner (Fig. 4B). PTH did not induce the expression of CYP27B1 mRNA in the kidney of normocalcemic sham-TPTX rats. The expression of CTR mRNA was not changed appreciably by the treatment with PTH and CT during the entire experimental period in the kidney of sham-TPTX rats.
Figure 4.
Time- and dose-dependent expression of CYP27B1, VDR, and CTR mRNAs in the kidney of sham-TPTX rats after CT and PTH injection. (A) Time-course experiments. Six-week-old rats fed a laboratory chow containing 2 units of vitamin D3 per gram of diet were injected with rat PTH-(1–34) subcutaneously at a dose of 5 μg per rat. CT was injected intramuscularly at a dose of 10 units per rat. (B) Dose–response experiments. Rats were killed 12 h after injection of 0.01–10 units of CT. Renal poly(A)+ RNA was purified from total RNA by using Oligotex dT 30, and Northern blots were probed with cDNAs for CYP27B1, VDR, and CTR. Similar results were obtained in four independent sets of experiments.
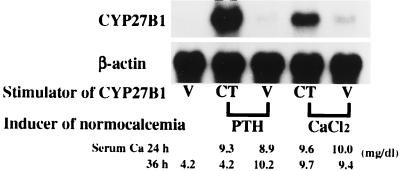
To examine whether the CT-induced transcription of CYP27B1 gene requires an adequate level of extracellular calcium, either PTH or CaCl2 was infused into TPTX rats to induce normocalcemia. Twenty-four hours after the initiation of PTH or CaCl2 infusion, CT was injected intramuscularly, and rats were killed 12 h later. Continuous injection of either PTH or CaCl2 per se had no effect on the expression of CYP27B1 mRNA in the kidney of TPTX rats (Fig. 5). Prior administration of either PTH or CaCl2 to induce normocalcemia greatly increased the CT-dependent expression of CYP27B1 mRNA in the kidney of TPTX rats.
Figure 5.
Effect of CT on the expression of CYP27B1 mRNA in the kidney of normocalcemic TPTX rats. Six-week-old rats were either sham-operated or TPTX under light ether anesthesia. Either CaCl2 or PTH was infused at a rate of 25 μmol/h or 25 ng/h for 36 h into TPTX rats fed a laboratory chow containing 2 units of vitamin D3 per gram of diet to induce normocalcemia. Serum levels of Ca attained a normal range 24 h after the initiation of PTH (8.9–9.3 mg/dl) and CaCl2 (9.6–10.0 mg/dl) infusion. Then, CT (10 units per rat) or vehicle (V: 0.5 μmol of sodium acetate per rat) was injected intramuscularly, and animals were killed 12 h later (36 h in total). Renal poly(A)+ RNA was purified from total RNA by using Oligotex dT 30, and Northern blots were probed with cDNAs for CYP27B1, VDR (not shown), and β-actin. Similar results were obtained in five independent sets of experiments.
To confirm that the CT-induced CYP27B1 is capable of catalyzing the hydroxylation of 25(OH)D3 at the 1α-position, we examined the in vivo metabolism of 25(OH)D3 in several rat models. The production of 1α,25(OH)2D3 was examined by an in vivo method using [3H]25(OH)D3 as a substrate. The in vivo synthesis of 1α,25(OH)2D3 was calculated from the rate of conversion of [3H]25(OH)D3 into [3H]1α,25(OH)2D3 and the serum concentration of 25(OH)D3. A single injection of CT into normocalcemic sham-TPTX rats greatly enhanced the in vivo conversion of [3H]25(OH)D3 into [3H]1α,25(OH)2D3 (Table 1). Similarly, a single intramuscular injection of CT into the normocalcemic TPTX rats generated by continuous infusion of PTH or CaCl2 elicited a marked increase in the conversion of [3H]25(OH)D3 into [3H]1α,25(OH)2D3. Six to 12 h after the injection of CT, 1α,25(OH)2D3 synthesis was greatly enhanced in sham-TPTX and normocalcemic TPTX rats, but not in hypocalcemic TPTX rats. The rate of conversion of [3H]25(OH)D3 into [3H]1α,25(OH)2D3 was not changed by the treatment with PTH in either TPTX or sham-TPTX rats. Administration of CT did not affect the serum concentration of 25(OH)D3 in sham-TPTX or TPTX rats. The rate of conversion of [3H]25(OH)D3 into [3H]1α,25(OH)2D3 was highest in rats fed a vitamin D-deficient diet, but these rat synthesized only a negligible amount (less than 4.5 pg/ml of serum per h) of 1α,25(OH)2D3 during the experimental period.
Table 1.
Serum levels of Ca and 25(OH)D3 and in vivo conversion of [3H]25(OH)D3 into [3H]1α,25(OH)2D3
| Operation | Dietary vitamin D3, units g of diet | Treatment | Serum 25(OH)D3, ng/dl | Conversion of [3H]25(OH)D3 into [3H]1α,25(OH)2D3, % per 6 h | Amount of 1α,25(OH)2D3 synthesized from 25(OH)D3, pg/ml of serum per h | ||
|---|---|---|---|---|---|---|---|
| Serum Ca level, mg/dl
| |||||||
| 24 h | 36 h | ||||||
| Sham | 2.0 | Vehicle | 10.1 ± 0.1 | 9.9 ± 1.4 | 39.9 ± 1.4 | 0.21 ± 0.02 | 14.0 |
| CT | 9.9 ± 0.2 | 6.3 ± 0.2 | 37.4 ± 2.4 | 2.30 ± 0.15 | 143.6 | ||
| PTH | 12.3 ± 0.2 | 12.8 ± 0.4 | 34.9 ± 4.5 | 0.24 ± 0.02 | 13.9 | ||
| TPTX | 2.0 | Vehicle | 4.9 ± 0.4 | 5.2 ± 0.2 | 37.3 ± 7.9 | 0.15 ± 0.04 | 9.3 |
| PTH | 10.3 ± 0.3 | 10.5 ± 0.2 | 35.1 ± 4.3 | 0.14 ± 0.02 | 8.3 | ||
| CT | 5.4 ± 0.2 | 4.4 ± 0.2 | 31.3 ± 2.9 | 0.18 ± 0.03 | 9.4 | ||
| PTH + CT | 9.9 ± 0.2 | 4.6 ± 0.1 | 38.9 ± 6.6 | 1.88 ± 0.11 | 122.0 | ||
| CaC12 | 10.3 ± 0.2 | 9.7 ± 0.2 | 32.8 ± 3.4 | 0.10 ± 0.04 | 5.6 | ||
| CaC12 + CT | 10.1 ± 0.2 | 9.5 ± 0.1 | 32.5 ± 4.2 | 1.72 ± 0.23 | 94.3 | ||
| Sham | 0 | Vehicle | — | 4.5 ± 0.3 | <2.0 | 13.58 ± 0.7 | <4.5 |
Five-week-old rats, either sham-operated or TPTX, were fed a laboratory chow containing 2 units of vitamin D3 per gram of diet. Either CaC12 or PTH was infused into TPTX rats at a rate of 25 μmol/h or 25 ng/h, respectively, for 36 h in total to induce normocalcemia. Twenty-four hours after the initiation of CaC12 or PTH infusion, CT (10 units per rat) or vehicle (0.5 μmol of sodium acetate per rat) was injected intramuscularly, and animals were killed 12 h later. [3H]25(OH)D3 was injected intravenously 6 h after the CT injection. Data were obtained from five rats. Values are expressed as the means ± SE. Male weanling rats were maintained for 3 weeks on a vitamin D-deficient diet containing 0.03% calcium and 0.6% phosphorus.
DISCUSSION
CYP27B1 is the only enzyme responsible for the biosynthesis of 1α,25(OH)2D3 from 25(OH)D3 (1, 2). Renal CYP27B1 activity is regulated by a number of physiological factors, including PTH, 1α,25(OH)2D3, CT, insulin, calcium, phosphorus, growth hormone, and prolactin (1, 2). Of these, PTH is known to be the most potent stimulator of CYP27B1. Most of the action of PTH in the kidney is mediated by the activation of membrane-associated adenylate cyclase, which leads to an increase in intracellular cAMP (21). The site of action of PTH along the nephron can be deduced by analyzing the distribution of PTH-sensitive adenylate cyclase. The renal 1α-hydroxylation is the most critical process in the activation of vitamin D (1–3). Horiuchi et al. (4) reported that CYP27B1 activity in the kidney decreased by 70–80% after TPTX in vitamin D-deficient rats, and the enzyme activity returned to a presurgical control level in response to exogenous PTH and cAMP. The maximal level of CYP27B1 stimulated by exogenous PTH or cAMP did not differ from the presurgical control level (4). The maximal effect of PTH together with cAMP was not additive (4). The present study clearly shows that the increased CYP27B1 activity is closely associated with the increased expression of CYP27B1 mRNA in the kidney (Fig. 2). These results indicate that, when the animal is in a vitamin D-deficient state, stimulation of CYP27B1 depends on secondary hyperparathyroidism and the enzyme activation is maximized by administering endogenous PTH. Furthermore, it is suggested that the PTH-induced expression of CYP27B1 mRNA is mediated by cAMP (Figs. 2 and 6).
Figure 6.
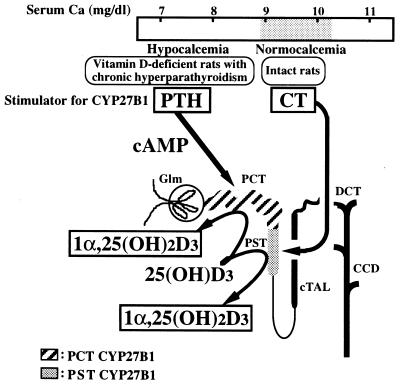
Stimulators and sites of 1α,25(OH)2D3 synthesis in the kidney of normocalcemic and hypocalcemic rats. CT stimulates CYP27B1 in the proximal straight tubules (PST) of normocalcemic rats. In contrast, PTH increases CYP27B1 in the proximal convoluted tubules (PCT) of rats fed a vitamin D-deficient or a vitamin D-replete low-Ca diet, which show severe secondary hyperparathyroidism. Glm, glomeruli; cTAL, cortical thick ascending limb; DCT, distal convoluted tubules; CCD, cortical collecting duct.
Using single-nephron segments dissected from vitamin D-deficient rats, Kawashima et al. (22) showed that CYP27B1 is present only in proximal convoluted tubules (PCT) and that the enzyme activity is reduced by 70% to 80% by TPTX (Fig. 6). These results, together with the selective localization of PTH-sensitive adenylate cyclase in the PCT, are consistent with the in vivo data of Horiuchi et al. (4) and the concept that the activation of CYP27B1 by PTH is mediated by cAMP.
Patients with primary hyperparathyroidism usually have a normal or a high serum level of 1α,25(OH)2D3 (23–25). The most important factor influencing the activity of renal CYP27B1 has been thought to be PTH. In a variety of in vitro and in vivo systems, PTH has been found to stimulate renal 1α-hydroxylase activity, which resulted in the production of 1α,25(OH)2D3 (26). Calcium ions may also play an important role in the control of the enzyme activity (27, 28). PTH is shown to directly stimulate the production of 1α,25(OH)2D3 in vivo (29) and in vitro (30). We previously reported that PTH stimulated CYP27B1 gene promoter-directed synthesis of luciferase by 17-fold, whereas forskolin stimulated it by only 3-fold in the PTH receptor-overexpressed AOK-B50 cells (14). Murayama et al. (15) also reported that not only PTH but also CT stimulated CYP27B1 gene promoter-directed synthesis of chloramphenicol acetyltransferase. However, the signaling pathway down-stream of these hormone receptors remains to be elucidated. Using reverse transcription–PCR (RT-PCR), St-Arnaud et al. (10) have reported that the infusion of a pharmacological dose of PTH (25 mg/kg per day) for 3 days increased the expression of CYP27B1 mRNA, but only 2-fold, in the kidney of normal mice. In the present study, a physiological dose of PTH had no effect on the expression of CYP27B1 mRNA in the kidney of hypocalcemic TPTX rats (Fig. 5). It is likely that the expression of renal CYP27B1 mRNA induced by a pharmacological dose of PTH in normal mice is mediated by the secondary stimulation of CT secretion.
In contrast to the PTH–vitamin D system, which acts primarily to prevent a fall in serum ionic calcium from the overall equilibrium level of 5 mg/dl, CT responds to a rise in serum ionic calcium. This probably does not occur spontaneously, since serum Ca always tends to decrease. The major action of CT appears to be to inhibit bone resorption, thus decreasing elevated serum Ca levels to normal. It has been shown that CT stimulates adenylate cyclase in bone and kidney. Using isolated single-nephron segments, Morel et al. (31) and Kawashima et al. (8) have independently demonstrated that CT-sensitive adenylate cyclase is localized in the distal nephron. Kawashima, et al. also reported that CT selectively stimulated CYP27B1 activity in proximal straight tubules (PST) (Fig. 6). Because CT had no effect on CYP27B1 activity of PCT in either intact or vitamin D-deficient TPTX rats, these results indicate that CT selectively stimulates CYP27B1 activity in the PST. The CT concentration in the circulation would probably be low in a vitamin D-deficient, hypocalcemic state, but serum CT is known to be elevated in the fetus. These results may explain why CYP27B1 activity was detected only in PCT of vitamin D-deficient rats, whereas the enzyme activity was present in both PCT and PST in vitamin D-replete fetuses (32). Because PST does not have CT-sensitive adenylate cyclase, it is likely that the action of CT to stimulate CYP27B1 is not mediated by cAMP. The activation of CYP27B1 by CT has been shown not only in vitamin D-deficient rats but also in vitamin D-replete rats (refs. 7 and 8 and the present study).
Galante et al. (5) and Lorenc et al. (6) have already reported that CT stimulates in vivo conversion of 25(OH)D3 into 1α,25(OH)2D3. Both groups employed vitamin D-deficient rats with intact thyroparathyroids. Lorenc et al. demonstrated that subcutaneous administration of CT every 8 h for 2 days increased the accumulation of [3H]1α,25(OH)2D3 from [3H]25(OH)D3 in plasma and intestine, and that the CT effect disappeared when parathyroid glands were removed. These results led them to conclude that the stimulatory effect of CT on 1α,25(OH)2D3 synthesis is mainly because of a secondary response of parathyroid glands. On the other hand, Horiuchi et al. (4) demonstrated that continuous infusion of CT together with CaCl2 into vitamin D-deficient TPTX rats enhanced in vivo accumulation of [3H]1α,25(OH)2D3 from [3H]25(OH)D3. Administration of CT to vitamin D-replete rats that had been TPTX thus raises circulating levels of 1α,25(OH)2D3. This effect of CT can be augmented in rats fed a high-Ca diet (5). At that time, the cause(s) of the discrepancy between the findings of Horiuchi et al. and those of Lorenc et al. was not clear. The present study clearly indicates that the concentration of extracellular calcium plays an important role in the renal expression of CYP27B1 mRNA by CT (Fig. 5). Nishioka et al. (33) provided evidence that CT plays a role in the postnatal increase of serum 1α,25(OH)2D3. Nesbitt et al. also reported that CT stimulated renal CYP27B1 activity in hypophosphatemic mice whose serum Ca level was within the normal range (34).
The biological significance of the stimulatory effects of CT on the 1α,25(OH)2D3 synthesis is not known. Plasma concentrations of 1α,25(OH)2D3 and CT have been reported to be significantly higher in lactating rats than in nonlactating rats (35, 36). In suckling rats (37) and in young rats (38), plasma CT concentrations tend to be increased. These animals all have a highly positive calcium balance. Thus it appears possible that CT is involved in the stimulation of renal biosynthesis of 1α,25(OH)2D3 in these animals. This hypothesis is supported by the present finding that PTH had no effect on the renal expression of CYP27B1 mRNA in vitamin D-replete intact rats (Figs. 4 and 6 and Table 1). We previously reported that down-regulation of VDR plays a key role in the production of 1α,25(OH)2D3 in renal PCT in a rat model of enhanced renal activity of CYP27B1 (20). In agreement with the previous report (20), administration of CT into sham-TPTX rats decreased the renal expression of VDR mRNA in a dose-dependent manner (Fig. 4). Therefore, the increased plasma concentration of 1α,25(OH)2D3 in lactating animals could be explained, at least in part, by the enhanced secretion of CT. The stimulation by CT of renal biosynthesis of 1α,25(OH)2D3 and the consequent enhancement of the intestinal absorption of calcium may reinforce this defense mechanism.
In conclusion, CT is a major regulator for the expression of the renal CYP27B1 gene in normocalcemic rats (Fig. 6). The relation between serum Ca levels and PTH/CT action in stimulating the CYP27B1 gene is of considerable interest and merits further investigation.
Acknowledgments
This study was supported by Grants–in–Aid (10307046, 08457494, and High-Technology Research Center Project) from the Ministry of Science, Sport, Education and Culture of Japan.
ABBREVIATIONS
- 25(OH)D3
25-hydroxyvitamin D3
- 1α,25(OH)2D3
1α,25-dihydroxyvitamin D3
- CYP27B1
25(OH)D3-1α-hydroxylase
- PTH
parathyroid hormone
- CT
calcitonin
- CTR
CT receptor
- TPTX
thyroparathyroidectomized or thyroparathyroidectomy
- VDR
vitamin D receptor
- PCT
proximal convoluted tubules
- PST
proximal straight tubules
References
- 1.DeLuca H F. FASEB J. 1988;2:224–236. [PubMed] [Google Scholar]
- 2.Suda T, Shinki T, Kurokawa K. Curr Opin Nephrol Hypertens. 1994;3:59–64. doi: 10.1097/00041552-199401000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Fraser D, Kodicek E. Nat New Biol. 1973;241:163–166. doi: 10.1038/newbio241163a0. [DOI] [PubMed] [Google Scholar]
- 4.Horiuchi N, Suda T, Takahashi H, Shimazawa E, Ogata E. Endocrinology. 1977;101:969–974. doi: 10.1210/endo-101-3-969. [DOI] [PubMed] [Google Scholar]
- 5.Galante L, Colston K W, MacAuley S J, MacIntyre I. Nature (London) 1972;238:271–273. doi: 10.1038/238271a0. [DOI] [PubMed] [Google Scholar]
- 6.Lorenc R, Tanaka Y, DeLuca H F, Jones G. Endocrinology. 1977;100:468–472. doi: 10.1210/endo-100-2-468. [DOI] [PubMed] [Google Scholar]
- 7.Horiuchi N, Takahashi H, Matsumoto T, Takahashi N, Shimazawa E, Suda T, Ogata E. Biochem J. 1979;184:269–275. doi: 10.1042/bj1840269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawashima H, Torikai S, Kurokawa K. Nature (London) 1981;291:327–329. doi: 10.1038/291327a0. [DOI] [PubMed] [Google Scholar]
- 9.Shinki T, Shimada H, Wakino S, Anazawa H, Hayashi M, Saruta T, DeLuca H F, Suda T. Proc Natl Acad Sci USA. 1997;94:12920–12925. doi: 10.1073/pnas.94.24.12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.St-Arnaud R, Messerlian S, Moir J M, Omdahl J L, Glorieux F H. J Bone Miner Res. 1997;12:1552–1559. doi: 10.1359/jbmr.1997.12.10.1552. [DOI] [PubMed] [Google Scholar]
- 11.Takeyama K, Kitanaka S, Sato T, Kobori M, Yanagisawa J, Kato S. Science. 1997;277:1827–1830. doi: 10.1126/science.277.5333.1827. [DOI] [PubMed] [Google Scholar]
- 12.Fu G K, Lin D, Zhang M Y H, Bikle D D, Shackleton C H L, Miller W L, Protale A A. Mol Endocrinol. 1997;11:1961–1970. doi: 10.1210/mend.11.13.0035. [DOI] [PubMed] [Google Scholar]
- 13.Monkawa T, Yoshida T, Wakino S, Shinki T, Anazawa H, DeLuca H F, Suda T, Hayashi M, Saruta T. Biochem Biophys Res Commun. 1997;239:527–533. doi: 10.1006/bbrc.1997.7508. [DOI] [PubMed] [Google Scholar]
- 14.Brenza H L, Kimmel-Jehan C, Jehan F, Shinki T, Wakino S, Anazawa H, Suda T, DeLuca H F. Proc Natl Acad Sci USA. 1998;95:1387–1391. doi: 10.1073/pnas.95.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murayama A, Takeyama K, Kitanaka S, Kodera Y, Hosoya T, Kato S. Biochem Biophys Res Commun. 1998;249:11–16. doi: 10.1006/bbrc.1998.9098. [DOI] [PubMed] [Google Scholar]
- 16.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 17.Feinberg A P, Vogelstein B. Anal Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- 18.Bligh E G, Dyer W J. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 19.Shinki T, Jin C H, Nishimura A, Nagai Y, Ohyama Y, Noshiro M, Okuda K, Suda T. J Biol Chem. 1992;267:13757–13762. [PubMed] [Google Scholar]
- 20.Iida K, Shinki T, Yamaguchi A, DeLuca H F, Kurokawa K, Suda T. Proc Natl Acad Sci USA. 1995;92:6112–6116. doi: 10.1073/pnas.92.13.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurokawa K. Kidney Int. 1987;32:760–771. doi: 10.1038/ki.1987.272. [DOI] [PubMed] [Google Scholar]
- 22.Kawashima H, Torikai S, Kurokawa K. Proc Natl Acad Sci USA. 1981;78:1199–1203. doi: 10.1073/pnas.78.2.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lund B, Sorenson O H, Lund B, Bishop J E, Norman A W. J Clin Endocrinol Metab. 1980;50:480–484. doi: 10.1210/jcem-50-3-480. [DOI] [PubMed] [Google Scholar]
- 24.Broadus A E, Horst R L, Lang R, Littledike E T, Rasmussen H. New Engl J Med. 1980;302:421–426. doi: 10.1056/NEJM198002213020801. [DOI] [PubMed] [Google Scholar]
- 25.Lalor B C, Mawer E B, Davies M, Lumb G A, Hunt L, Adams P H. Clin Sci. 1989;76:81–86. doi: 10.1042/cs0760081. [DOI] [PubMed] [Google Scholar]
- 26.Kumar R. Endocr Rev. 1989;1:258–267. doi: 10.1210/edrv-1-3-258. [DOI] [PubMed] [Google Scholar]
- 27.Trechsel U, Eisman J A, Fischer J A, Bonjour J P, Fleisch H. Am J Physiol. 1980;239:E119–E124. doi: 10.1152/ajpendo.1980.239.2.E119. [DOI] [PubMed] [Google Scholar]
- 28.Larkins R G, Colston K W, Galante L S, MacAuley S J, Evans I M A, MacIntyre I. Lancet. 1973;ii:289–291. doi: 10.1016/s0140-6736(73)90794-0. [DOI] [PubMed] [Google Scholar]
- 29.Omdahl J L. J Biol Chem. 1978;253:8474–8478. [PubMed] [Google Scholar]
- 30.Rost C R, Bikle D D, Kaplan R A. Endocrinology. 1981;108:1002–1006. doi: 10.1210/endo-108-3-1002. [DOI] [PubMed] [Google Scholar]
- 31.Morel F. Am J Physiol. 1981;240:F159–F164. doi: 10.1152/ajprenal.1981.240.3.F159. [DOI] [PubMed] [Google Scholar]
- 32.Akiba T, Endo H, Koseki C, Sakai F, Horiuchi N, Suda T. Biochem Biophys Res Commun. 1989;94:313–318. doi: 10.1016/s0006-291x(80)80222-1. [DOI] [PubMed] [Google Scholar]
- 33.Nishioka T, Yasuda T, Niimi H, Nakajima H. Eur J Pediatr. 1988;147:148–152. doi: 10.1007/BF00442212. [DOI] [PubMed] [Google Scholar]
- 34.Nesbitt T, Lobaugh B, Drezner M. J Clin Invest. 1987;79:15–19. doi: 10.1172/JCI112776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar R, Cohen W R, Silva P, Epstein F H. J Clin Invest. 1979;63:342–344. doi: 10.1172/JCI109308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pike J W, Parker J B, Haussler M R, Boass A, Toverud S D. Science. 1979;204:1427–1429. doi: 10.1126/science.451573. [DOI] [PubMed] [Google Scholar]
- 37.Cooper C W, Obie J F, Toverud S U, Munson P L. Endocrinology. 1977;101:1657–1664. doi: 10.1210/endo-101-6-1657. [DOI] [PubMed] [Google Scholar]
- 38.Talmage R V, Doppelt S H, Cooper C W. Proc Soc Exp Biol Med. 1975;149:855–859. doi: 10.3181/00379727-149-38913. [DOI] [PubMed] [Google Scholar]