Abstract
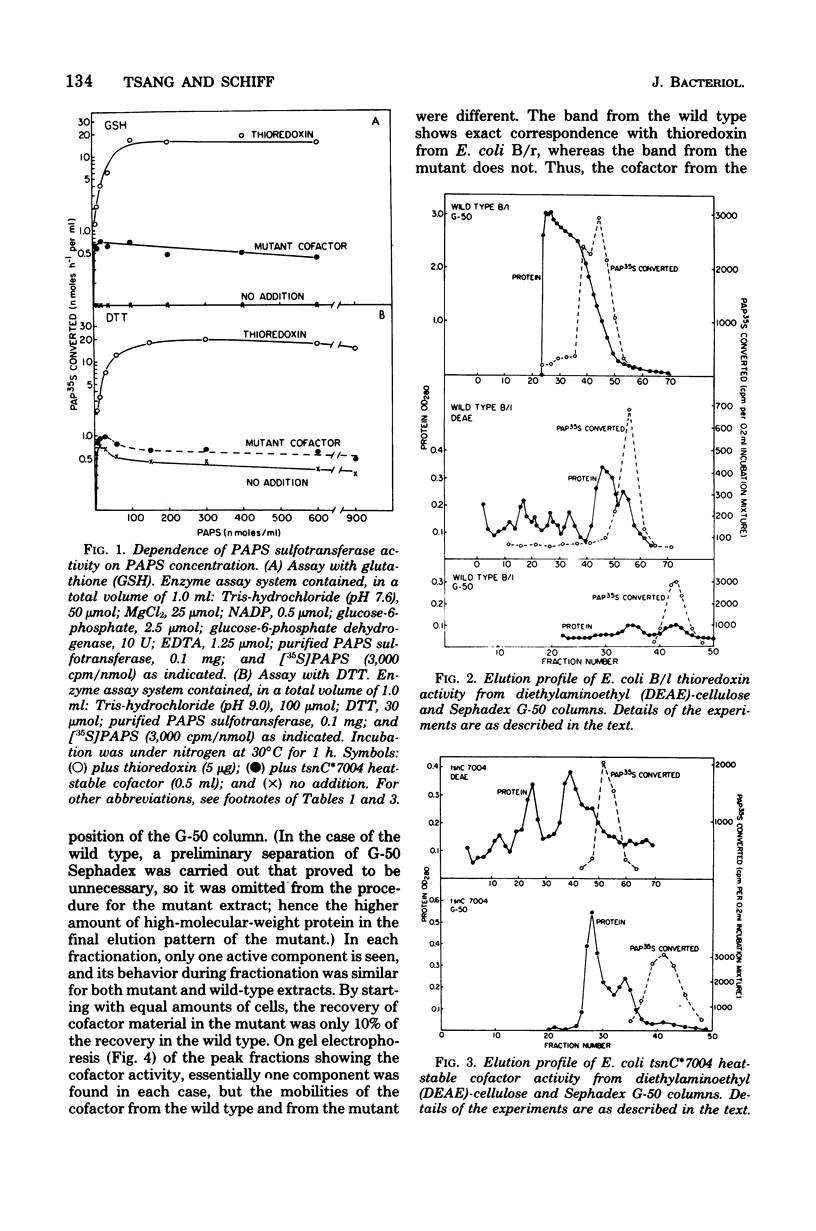
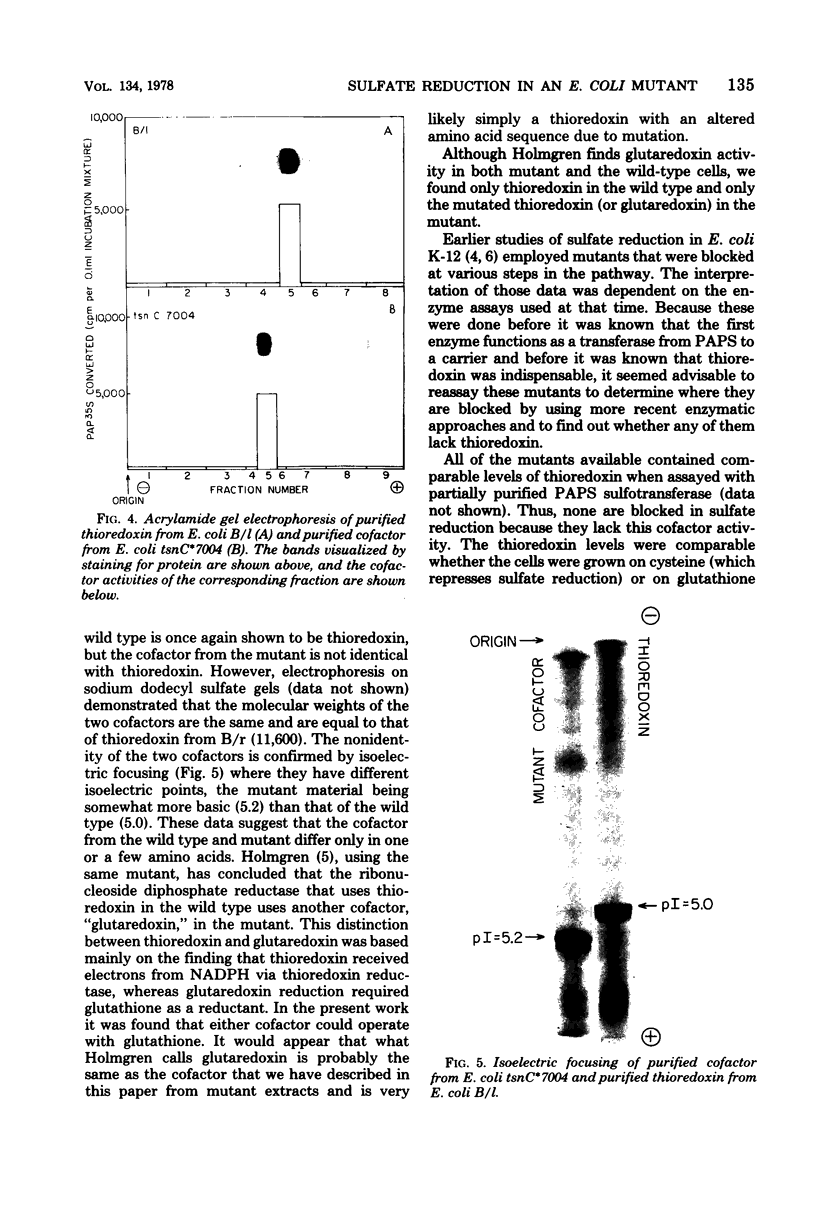
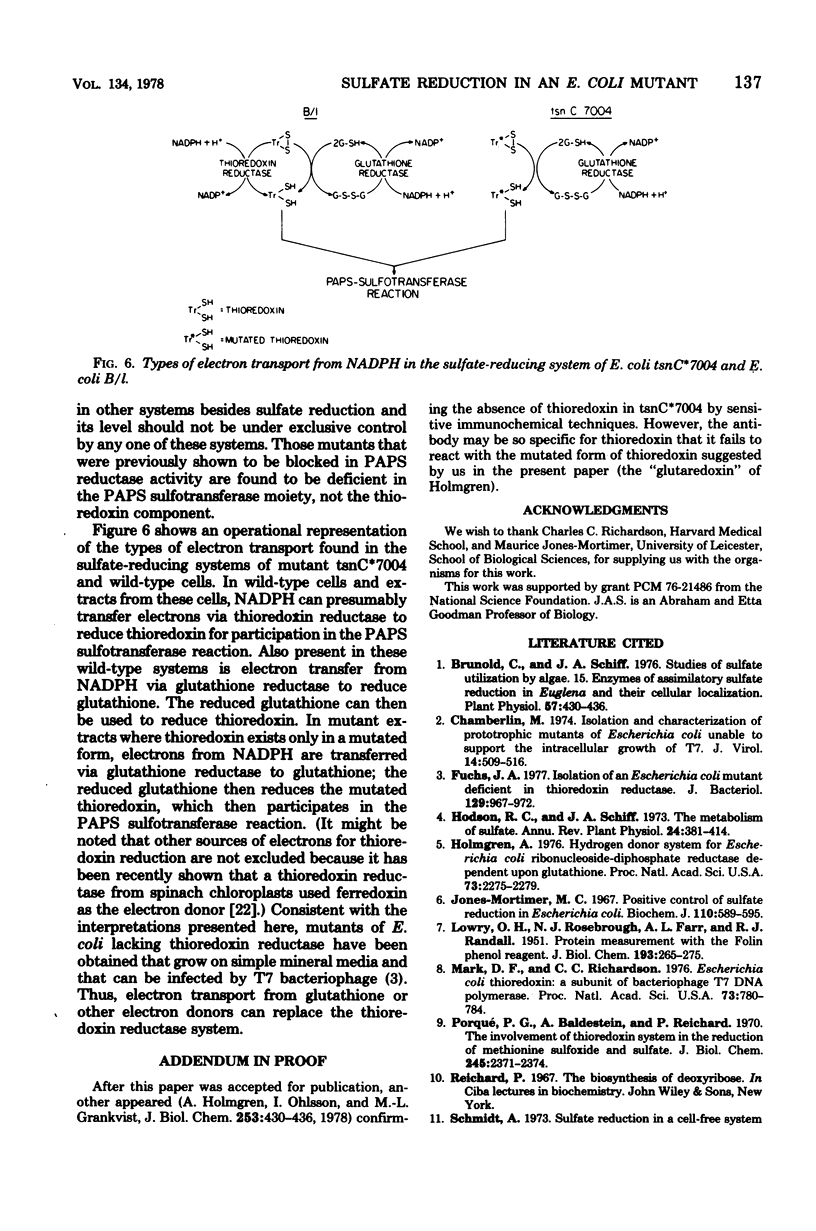
An investigation of sulfate reduction in B tsnC*7004, a mutant of Escherichia coli lacking thioredoxin, is reported. Although thioredoxin is indispensable for the adenosine 3'-phosphate 5'-phosphosulfate (PAPS) sulfotransferase reaction under the usual conditions of assay in extracts of wild-type cells, the mutant grew as well as the wild type on sulfate, indicating that sulfate reduction is not rate limiting for growth. Another cofactor for the PAPS sulfotransferase reaction was found in extracts of the mutant that is absent from wild type cells. This cofactor was indistinguishable from thioredoxin in molecular weight but had a slightly different isoelectric point, allowing a separation of the two types of molecules by isoelectric focusing. Whereas electrons from nicotinamide adenine dinucleotide phosphate, reduced form, could be transferred via thioredoxin reductase or via glutathione and glutathione reductase to reduce thioredoxin in extracts of wild-type cells, electrons from nicotinamide adenine dinucleotide, reduced form, could only be transferred to the cofactor of the mutant via glutathione and glutathione reductase. All of the other available mutants blocked in sulfate reduction in E. coli contained normal levels of thioredoxin. The "PAPS reductase" mutant is shown to be blocked in the PAPS sulfotransferase reaction. We conclude that the cofactor found in mutant B tsnC*7004 is probably a mutated thioredoxin with an amino acid substitution that alters the isoelectric point and the reactivity with thioredoxin reductase.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunold C., Schiff J. A. Studies of sulfate utilization of algae: 15. Enzymes of assimilatory sulfate reduction in euglena and their cellular localization. Plant Physiol. 1976 Mar;57(3):430–436. doi: 10.1104/pp.57.3.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin M. Isolation and characterization of prototrophic mutants of Escherichia coli unable to support the intracellular growth of T7. J Virol. 1974 Sep;14(3):509–516. doi: 10.1128/jvi.14.3.509-516.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs J. Isolation of an Escherichia coli mutant deficient in thioredoxin reductase. J Bacteriol. 1977 Feb;129(2):967–972. doi: 10.1128/jb.129.2.967-972.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez Porqué P., Baldesten A., Reichard P. The involvement of the thioredoxin system in the reduction of methionine sulfoxide and sulfate. J Biol Chem. 1970 May 10;245(9):2371–2374. [PubMed] [Google Scholar]
- Holmgren A. Hydrogen donor system for Escherichia coli ribonucleoside-diphosphate reductase dependent upon glutathione. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2275–2279. doi: 10.1073/pnas.73.7.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A., Ohlsson I., Grankvist M. L. Thiroedoxin from Escherichia coli. Radioimmunological and enzymatic determinations in wild type cells and mutants defective in phage T7 DNA replication. J Biol Chem. 1978 Jan 25;253(2):430–436. [PubMed] [Google Scholar]
- Jones-Mortimer M. C. Positive control of sulphate reduction in Escherichia coli. Isolation, characterization and mapping oc cysteineless mutants of E. coli K12. Biochem J. 1968 Dec;110(3):589–595. doi: 10.1042/bj1100589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lik-Shing Tsang M., Schiff J. A. Properties of enzyme fraction A from Chlorella and copurification of 3' (2'), 5'-biphosphonucleoside 3' (2')-phosphohydrolase, adenosine 5'phosphosulfate sulfohydrolase and adenosine-5'-phosphosulfate cyclase activities. Eur J Biochem. 1976 May 17;65(1):113–121. doi: 10.1111/j.1432-1033.1976.tb10395.x. [DOI] [PubMed] [Google Scholar]
- Mark D. F., Richardson C. C. Escherichia coli thioredoxin: a subunit of bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1976 Mar;73(3):780–784. doi: 10.1073/pnas.73.3.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A., Abrams W. R., Schiff J. A. Reduction of adenosine 5'-phosphosulfate to cysteine in extracts from Chlorella and mutants blocked for sulfate reduction. Eur J Biochem. 1974 Sep 16;47(3):423–434. doi: 10.1111/j.1432-1033.1974.tb03709.x. [DOI] [PubMed] [Google Scholar]
- Schmidt A. Sulfate reduction in a cell-free system of Chlorella. The ferredoxin dependent reduction of a protein-bound intermediate by a thiosulfonate reductase. Arch Mikrobiol. 1973 Oct 4;93(1):29–52. [PubMed] [Google Scholar]
- Siegel L. M., Murphy M. J., Kamin H. Reduced nicotinamide adenine dinucleotide phosphate-sulfite reductase of enterobacteria. I. The Escherichia coli hemoflavoprotein: molecular parameters and prosthetic groups. J Biol Chem. 1973 Jan 10;248(1):251–264. [PubMed] [Google Scholar]
- Tsang M. L., Lemieux J., Schiff J. A., Bojarski T. B. Preparation of adenosine 5'-phosphosulfate (APS) from adenosine 3'-phosphate 5'-phosphosulfate (PAPS) prepared by an improved procedure. Anal Biochem. 1976 Aug;74(2):623–626. doi: 10.1016/0003-2697(76)90249-9. [DOI] [PubMed] [Google Scholar]
- Tsang M. L., Schiff J. A. Sulfate-reducing pathway in Escherichia coli involving bound intermediates. J Bacteriol. 1976 Mar;125(3):923–933. doi: 10.1128/jb.125.3.923-933.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON L. G., ASAHI T., BANDURSKI R. S. Yeast sulfate-reducing system. I. Reduction of sulfate to sulfite. J Biol Chem. 1961 Jun;236:1822–1829. [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Wilson L. G., Bierer D. The formation of exchangeable sulphite from adenosine 3'-phosphate 5'-sulphatophosphate in yeast. Biochem J. 1976 Aug 15;158(2):255–270. doi: 10.1042/bj1580255. [DOI] [PMC free article] [PubMed] [Google Scholar]




