Abstract
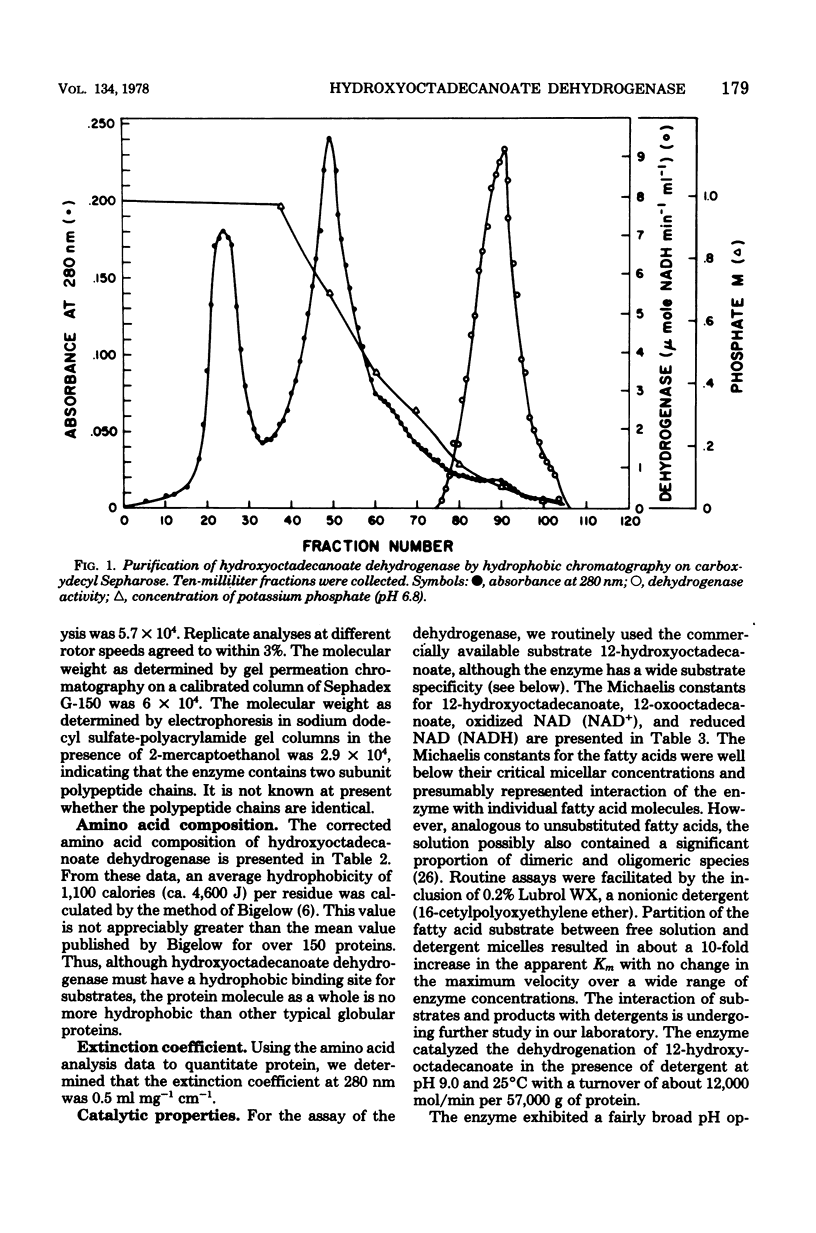
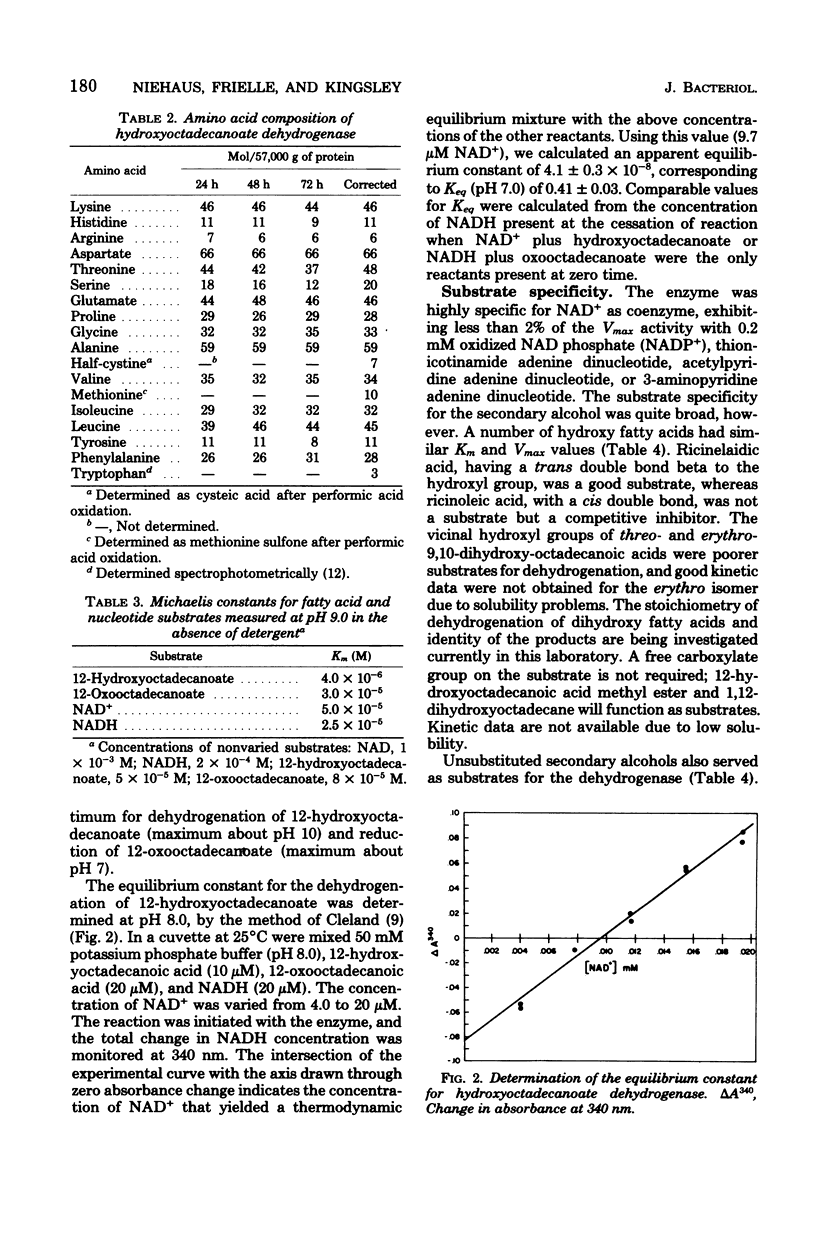
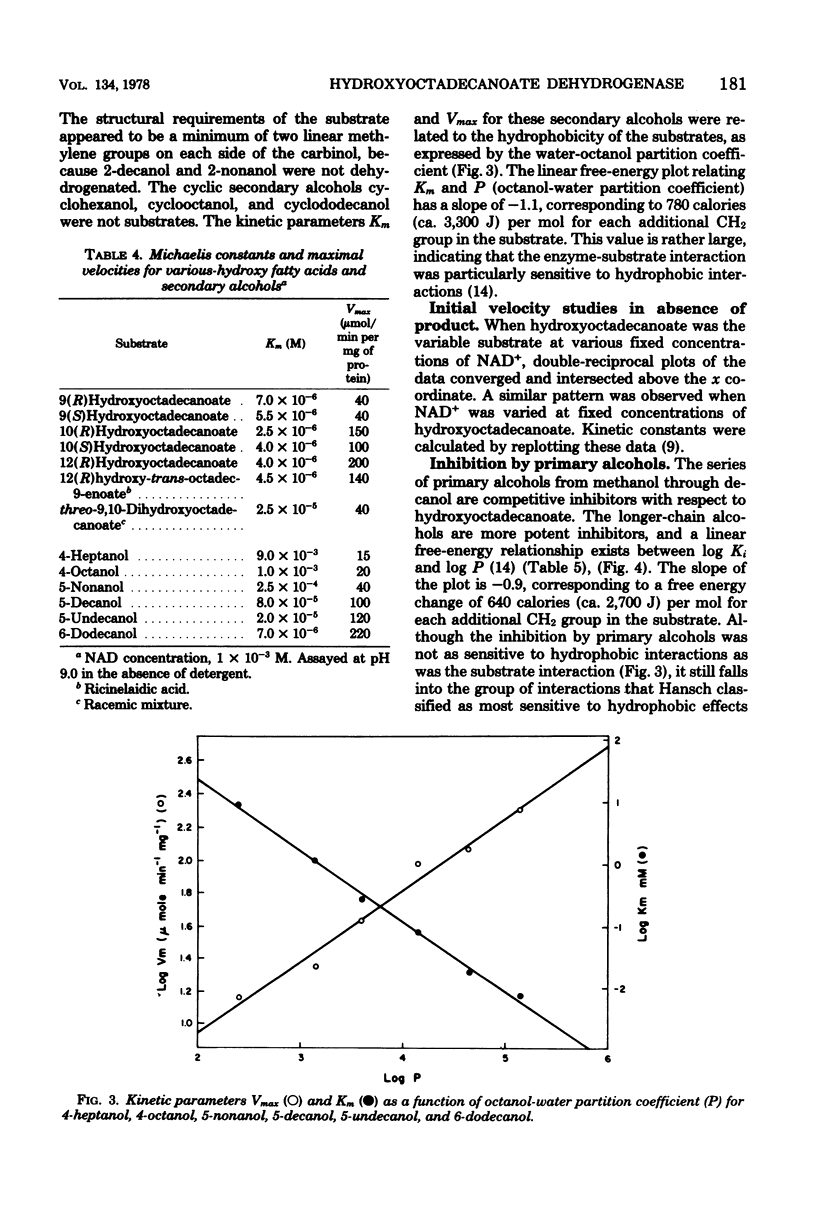
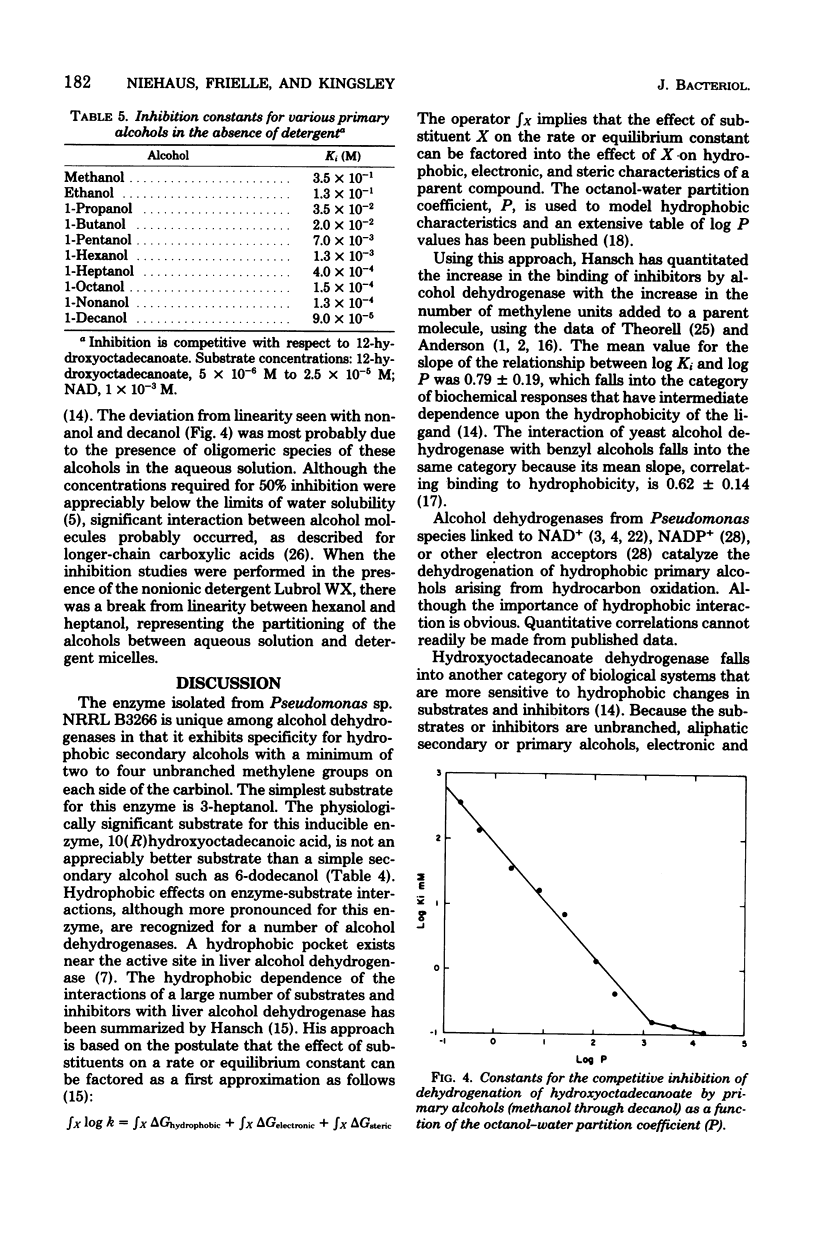
Growth of Pseudomonas sp. NRRL B3266 in the presence of oleic acid resulted in the induction of two enzymes: oleate hydratase, which produced 10(R)hydroxyoctadecanoate, and hydroxyoctadecanoate dehydrogenase, which catalyzed the oxidized nicotinamide adenine dinucleotide-dependent production of 10-oxooctadecanoate. This latter enzyme was purified to homogeneity and shown to consist of two polypeptide chains of about 29,000 daltons each. The enzyme had a broad substrate specificity, catalyzing the dehydrogenation of a number of 18-carbon hydroxy fatty acids. The kinetic parameters for various 10- and 12-hydroxy fatty acids were similar (Km ca. 5 micron and Vmax ca. 50 to 200 mumol/min per mg of protein). The enzyme also catalyzed the dehydrogenation of unsubstituted secondary alcohols. The effectiveness of these alcohols as substrates was highly dependent on their hydrophobicity, the Km decreasing from 9 mM for 4-heptanol to 7 micron for 6-dodecanol. Inhibition of the enzyme by primary alcohols also showed a dependence on hydrophobicity, the Ki decreasing from 350 mM for methanol to 90 micron for decanol.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON B. M., REYNOLDS M. L. INHIBITION OF YEAST ALCOHOL DEHYDROGENASE BY ALKYLAMMONIUM CHLORIDES. Biochim Biophys Acta. 1965 Jan;96:45–51. doi: 10.1016/0005-2787(65)90607-6. [DOI] [PubMed] [Google Scholar]
- AZOULAY E., HEYDEMAN M. T. Extraction and properties of alcohol dehydrogenase from Pseudomonas aeruginosa. Biochim Biophys Acta. 1963 May 7;73:1–6. doi: 10.1016/0006-3002(63)90353-6. [DOI] [PubMed] [Google Scholar]
- BAPTIST J. N., GHOLSON R. K., COON M. J. Hydrocarbon oxidation by a bacterial enzyme system. I. Products of octane oxidation. Biochim Biophys Acta. 1963 Jan 1;69:40–47. doi: 10.1016/0006-3002(63)91223-x. [DOI] [PubMed] [Google Scholar]
- Bigelow C. C. On the average hydrophobicity of proteins and the relation between it and protein structure. J Theor Biol. 1967 Aug;16(2):187–211. doi: 10.1016/0022-5193(67)90004-5. [DOI] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Hansch C., Dunn W. J., 3rd Linear relationships between lipophilic character and biological activity of drugs. J Pharm Sci. 1972 Jan;61(1):1–19. doi: 10.1002/jps.2600610102. [DOI] [PubMed] [Google Scholar]
- Hansch C., Schaeffer J., Kerley R. Alcohol dehydrogenase structure-activity relationships. J Biol Chem. 1972 Jul 25;247(14):4703–4710. [PubMed] [Google Scholar]
- Heitz J. R., Anderson C. D., Anderson B. M. Inactivation of yeast alcohol dehydrogenase by N-alkylmaleimides. Arch Biochem Biophys. 1968 Sep 20;127(1):627–636. doi: 10.1016/0003-9861(68)90271-3. [DOI] [PubMed] [Google Scholar]
- Mortimer C. E., Niehaus W. G., Jr Enzymatic interconversion of oleic acid, 10-hydroxyoctadecanoic acid, and trans-delta 10-octadecenoic acid. Reaction pathway and stereospecificity. J Biol Chem. 1974 May 10;249(9):2833–2842. [PubMed] [Google Scholar]
- Niehaus W. G., Jr, Schroepfer G. J., Jr The reversible hydration of oleic acid to 10D-hydroxystearic acid. Biochem Biophys Res Commun. 1965 Nov 8;21(3):271–275. doi: 10.1016/0006-291x(65)90282-2. [DOI] [PubMed] [Google Scholar]
- Niehaus W. G., Jr, Torkelson A., Kisic A., Bednarczyk D. J., Schroepfer G. J., Jr Stereospecific hydration of the delta-9 double bond of oleic acid. J Biol Chem. 1970 Aug 10;245(15):3790–3797. [PubMed] [Google Scholar]
- Penniston J. T., Beckett L., Bentley D. L., Hansch C. Passive permeation of organic compounds through biological tissue: a non-steady-state theory. Mol Pharmacol. 1969 Jul;5(4):333–341. [PubMed] [Google Scholar]
- Schöpp W., Grunow M., Tauchert H., Aurich H. Specific hydrophobic chromatography of alcohol dehydrogenases on 10-carboxydecyl-Sepharose. FEBS Lett. 1976 Oct 1;68(2):198–202. doi: 10.1016/0014-5793(76)80435-8. [DOI] [PubMed] [Google Scholar]
- Shore J. D., Theorell H. Substrate inhibition effects in the liver alcohol dehydrogenase reaction. Arch Biochem Biophys. 1966 Nov;117(2):375–380. doi: 10.1016/0003-9861(66)90425-5. [DOI] [PubMed] [Google Scholar]
- Smith R., Tanford C. Hydrophobicity of Long Chain n-Alkyl Carboxylic Acids, as Measured by Their Distribution Between Heptane and Aqueous Solutions. Proc Natl Acad Sci U S A. 1973 Feb;70(2):289–293. doi: 10.1073/pnas.70.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]
- van der Linden A. C., v Ravenswaay Claasen J. C. Hydrophobic enzymes in hydrocarbon degradation. Lipids. 1971 Jul;6(7):437–443. doi: 10.1007/BF02531225. [DOI] [PubMed] [Google Scholar]


