Abstract
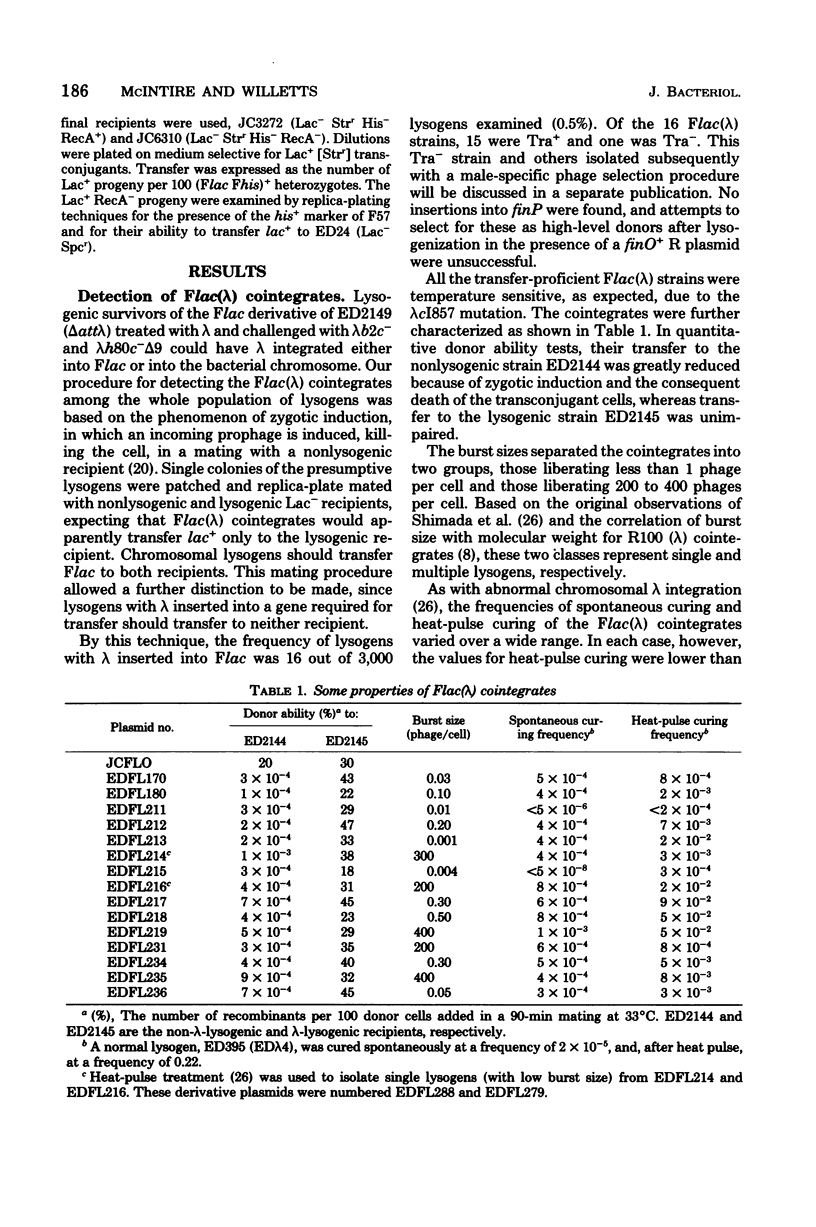
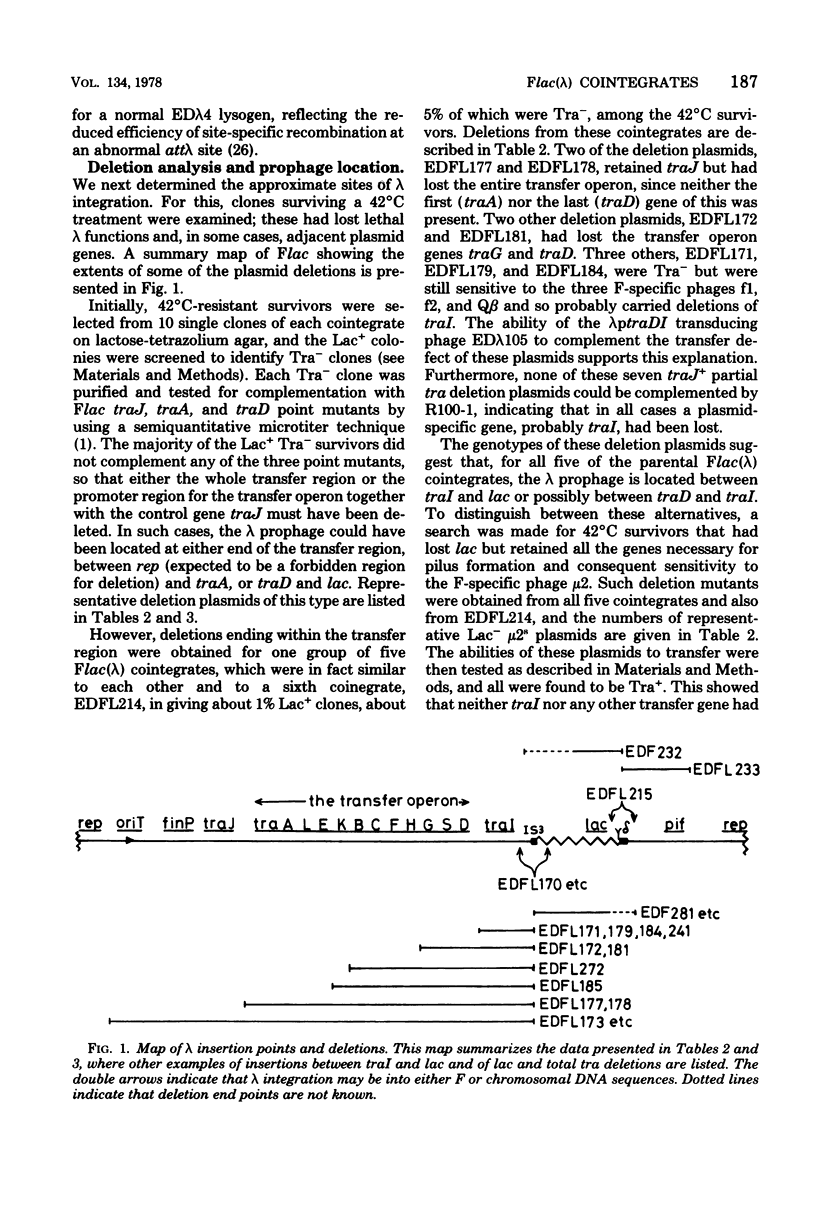
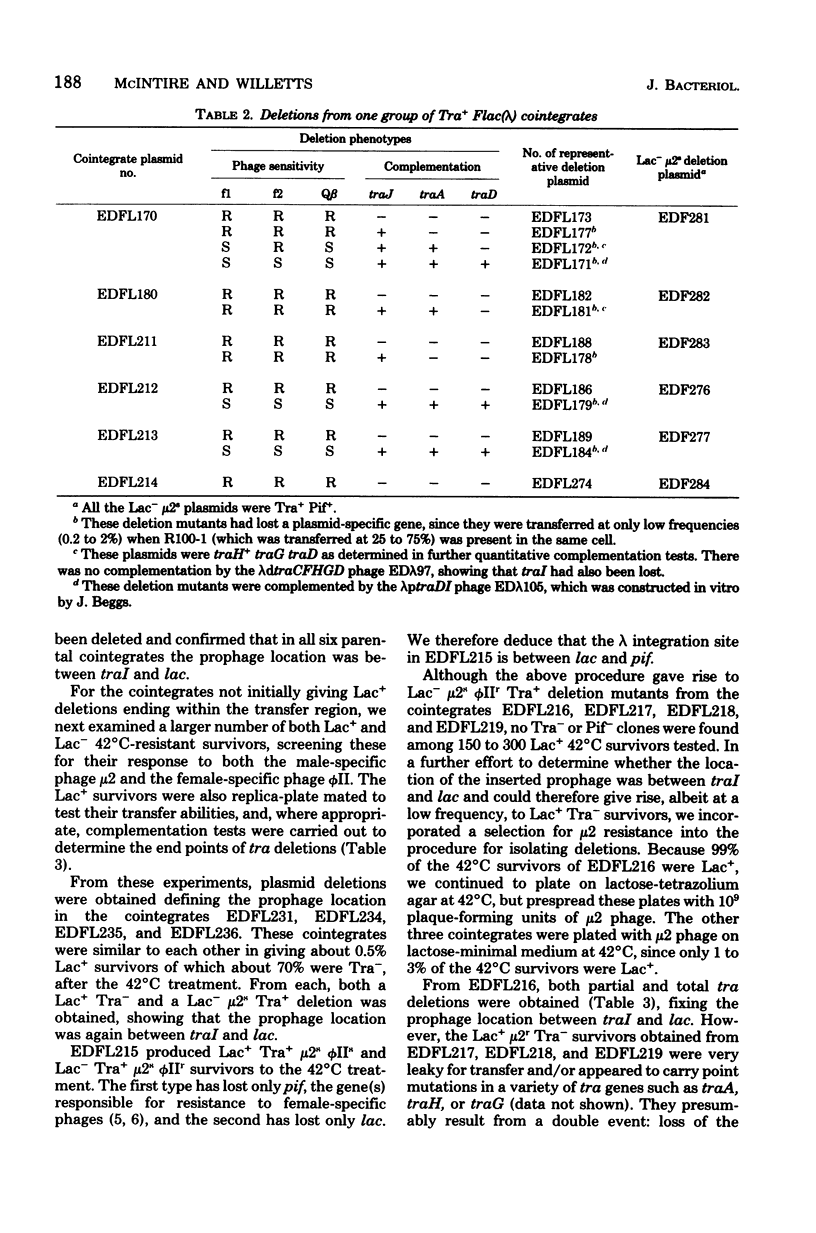
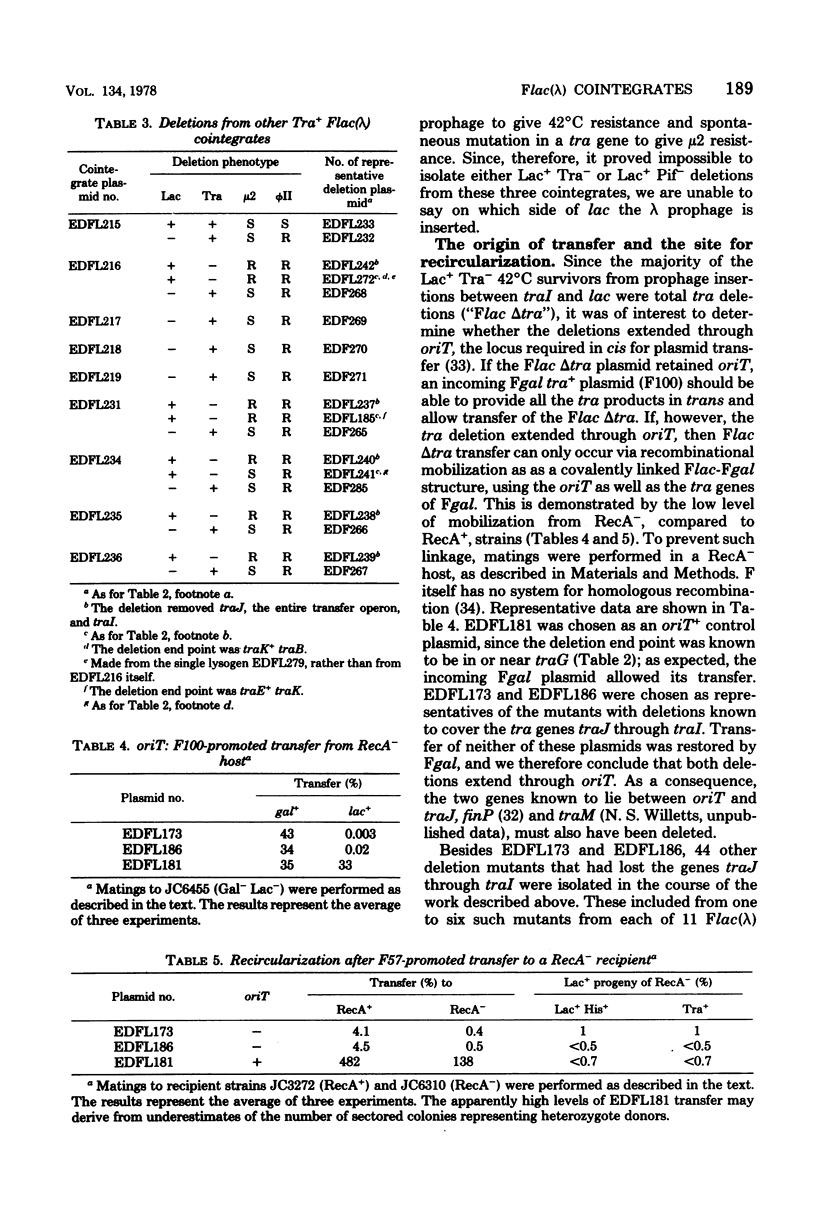
Fifteen cointegrates of the plasmid Flac and prophage lambda that had suffered no detectable change in plasmid phenotype were isolated and characterized. The locations of the prophage insertions were determined by genetic analysis of deletion mutants obtained from each cointegrate as survivors of growth at 42 degrees C. In 11 cointegrates, the prophage was inserted between traI and lac, although probably in more than one location; in 3 others, it was on one side or the other of lac; and in 1 it was between lac and pif. Deletions covering all or part of the transfer region, as well as of lac and of pif, were obtained in the course of this analysis. Deletion mutants that had lost all known transfer genes were also oriT, but they retained the capacity to recircularize after transfer. Attempts were made to isolate lambda transducing phages for nearby plasmid genes from the cointegrates, and lambdaptraGD, lambdaptraD, lambdaptraI, and lambdadtraDI phages were obtained.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M. A semi-automatic technique for conducting many bacterial matings concurrently. Genet Res. 1971 Jun;17(3):261–266. doi: 10.1017/s0016672300012283. [DOI] [PubMed] [Google Scholar]
- Achtman M., Willetts N., Clark A. J. Beginning a genetic analysis of conjugational transfer determined by the F factor in Escherichia coli by isolation and characterization of transfer-deficient mutants. J Bacteriol. 1971 May;106(2):529–538. doi: 10.1128/jb.106.2.529-538.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Willetts N., Clark A. J. Conjugational complementation analysis of transfer-deficient mutants of Flac in Escherichia coli. J Bacteriol. 1972 Jun;110(3):831–842. doi: 10.1128/jb.110.3.831-842.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg D. D., Mabie C. T., Malamy M. H. T7 protein synthesis in F-factor-containing cells: evidence for an episomally induced impairment of translation and relation to an alteration in membrane permeability. J Virol. 1975 Jan;17(1):94–105. doi: 10.1128/jvi.17.1.94-105.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton J. R., Haselkorn R. Permeability lesions in male Escherichia coli infected with bacteriophage T7. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2222–2226. doi: 10.1073/pnas.72.6.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK A. J., MARGULIES A. D. ISOLATION AND CHARACTERIZATION OF RECOMBINATION-DEFICIENT MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1965 Feb;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey W. B., Willetts N. S. Plasmid co-integrates of prophage lambda and R factor R100. J Bacteriol. 1976 Apr;126(1):166–176. doi: 10.1128/jb.126.1.166-176.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkow S., Tompkins L. S., Silver R. P., Guerry P., Le Blanc D. J. The problems of drug-resistant pathogenic bacteria. The replication of R-factor DNA in Escherichia coli K-12 following conjugation. Ann N Y Acad Sci. 1971 Jun 11;182:153–171. doi: 10.1111/j.1749-6632.1971.tb30654.x. [DOI] [PubMed] [Google Scholar]
- Fenwick R. G., Jr, Curtiss R., 3rd Conjugal deoxyribonucleic acid replication by Escherichia coli K-12: stimulation in dnaB(ts) donors by minicells. J Bacteriol. 1973 Dec;116(3):1212–1223. doi: 10.1128/jb.116.3.1212-1223.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan D., Willetts N. The site of action of the F transfer inhibitor. Mol Gen Genet. 1973 Dec 31;127(4):307–316. doi: 10.1007/BF00267101. [DOI] [PubMed] [Google Scholar]
- Gasson M. J., Willetts N. S. Five control systems preventing transfer of Escherichia coli K-12 sex factor F. J Bacteriol. 1975 May;122(2):518–525. doi: 10.1128/jb.122.2.518-525.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W., Dressler D. DNA replication: the rolling circle model. Cold Spring Harb Symp Quant Biol. 1968;33:473–484. doi: 10.1101/sqb.1968.033.01.055. [DOI] [PubMed] [Google Scholar]
- Guyer M. S., Clark A. J. cis-Dominant, transfer-deficient mutants of the Escherichia coli K-12 F sex factor. J Bacteriol. 1976 Jan;125(1):233–247. doi: 10.1128/jb.125.1.233-247.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmuth R., Achtman M. Operon structure of DNA transfer cistrons on the F sex factor. Nature. 1975 Oct 23;257(5528):652–656. doi: 10.1038/257652a0. [DOI] [PubMed] [Google Scholar]
- Hu S., Ohtsubo E., Davidson N. Electron microscopic heteroduplex studies of sequence relations among plasmids of Escherichia coli: structure of F13 and related F-primes. J Bacteriol. 1975 May;122(2):749–763. doi: 10.1128/jb.122.2.749-763.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Otsubo E., Davidson N., Saedler H. Electron microscope heteroduplex studies of sequence relations among bacterial plasmids: identification and mapping of the insertion sequences IS1 and IS2 in F and R plasmids. J Bacteriol. 1975 May;122(2):764–775. doi: 10.1128/jb.122.2.764-775.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Ptashne K., Cohen S. N., Davidson N. alphabeta sequence of F is IS31. J Bacteriol. 1975 Aug;123(2):687–692. doi: 10.1128/jb.123.2.687-692.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ippen-Ihler K., Achtman M., Willetts N. Deletion map of the Escherichia coli K-12 sex factor F: the order of eleven transfer cistrons. J Bacteriol. 1972 Jun;110(3):857–863. doi: 10.1128/jb.110.3.857-863.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., WOLLMAN E. Induction spontanée du développement du bactériophage lambda au cours de la recombinaison génétique, chez Escherichia coli K 12. C R Hebd Seances Acad Sci. 1954 Jul 19;239(3):317–319. [PubMed] [Google Scholar]
- Lovett M. A., Helinski D. R. Method for the isolation of the replication region of a bacterial replicon: construction of a mini-F'kn plasmid. J Bacteriol. 1976 Aug;127(2):982–987. doi: 10.1128/jb.127.2.982-987.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin D. A., Hohn B. Filamentous bacterial viruses. Bacteriol Rev. 1969 Jun;33(2):172–209. doi: 10.1128/br.33.2.172-209.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara K. Properties of sex factor and related episomes isolated from purified Escherichia coli zygote cells. J Mol Biol. 1968 Nov 28;38(1):89–108. doi: 10.1016/0022-2836(68)90130-7. [DOI] [PubMed] [Google Scholar]
- Ohki M., Tomizawa J. Asymmetric transfer of DNA strands in bacterial conjugation. Cold Spring Harb Symp Quant Biol. 1968;33:651–658. doi: 10.1101/sqb.1968.033.01.074. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Hsu M. T., Otsubo E., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. I. Structure of F-prime factors. J Mol Biol. 1972 Nov 14;71(2):471–497. doi: 10.1016/0022-2836(72)90363-4. [DOI] [PubMed] [Google Scholar]
- Shimada K., Weisberg R. A., Gottesman M. E. Prophage lambda at unusual chromosomal locations. I. Location of the secondary attachment sites and the properties of the lysogens. J Mol Biol. 1972 Feb 14;63(3):483–503. doi: 10.1016/0022-2836(72)90443-3. [DOI] [PubMed] [Google Scholar]
- Skurray R. A., Guyer M. S., Timmis K., Cabello F., Cohen S. N., Davidson N., Clark A. J. Replication region fragments cloned from Flac+ are identical to EcoRI fragment f5 of F. J Bacteriol. 1976 Sep;127(3):1571–1575. doi: 10.1128/jb.127.3.1571-1575.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurray R. A., Willetts N., Reeves P. Effect of tra mutations on F factor-specified immunity to lethal zygosis. Mol Gen Genet. 1976 Jul 23;146(2):161–165. doi: 10.1007/BF00268084. [DOI] [PubMed] [Google Scholar]
- Timmis K., Cabello F., Cohen S. N. Cloning, isolation, and characterization of replication regions of complex plasmid genomes. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2242–2246. doi: 10.1073/pnas.72.6.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S. Location of the origin of transfer of the sex factor F. J Bacteriol. 1972 Nov;112(2):773–778. doi: 10.1128/jb.112.2.773-778.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S. Recombination and the Escherichia coli K-12 sex factor F. J Bacteriol. 1975 Jan;121(1):36–43. doi: 10.1128/jb.121.1.36-43.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N., Achtman M. Genetic analysis of transfer by the Escherichia coli sex factor F, using P1 transductional complementation. J Bacteriol. 1972 Jun;110(3):843–851. doi: 10.1128/jb.110.3.843-851.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N., Bastarrachea F. Genetic and physicochemical characterization of Escherichia coli strains carrying fused F' elements derived from KLF1 and F57. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1481–1485. doi: 10.1073/pnas.69.6.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. Mapping loci for surface exclusion and incompatibility on the F factor of Escherichia coli K-12. J Bacteriol. 1974 Jun;118(3):778–782. doi: 10.1128/jb.118.3.778-782.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N., Maule J., McIntire S. The genetic locations of traO, finP and tra-4 on the E. coli K12 sex factor F. Genet Res. 1975 Dec;26(3):255–263. doi: 10.1017/s0016672300016050. [DOI] [PubMed] [Google Scholar]
- Willetts N. The transcriptional control of fertility in F-like plasmids. J Mol Biol. 1977 May 5;112(1):141–148. doi: 10.1016/s0022-2836(77)80161-7. [DOI] [PubMed] [Google Scholar]


