Abstract
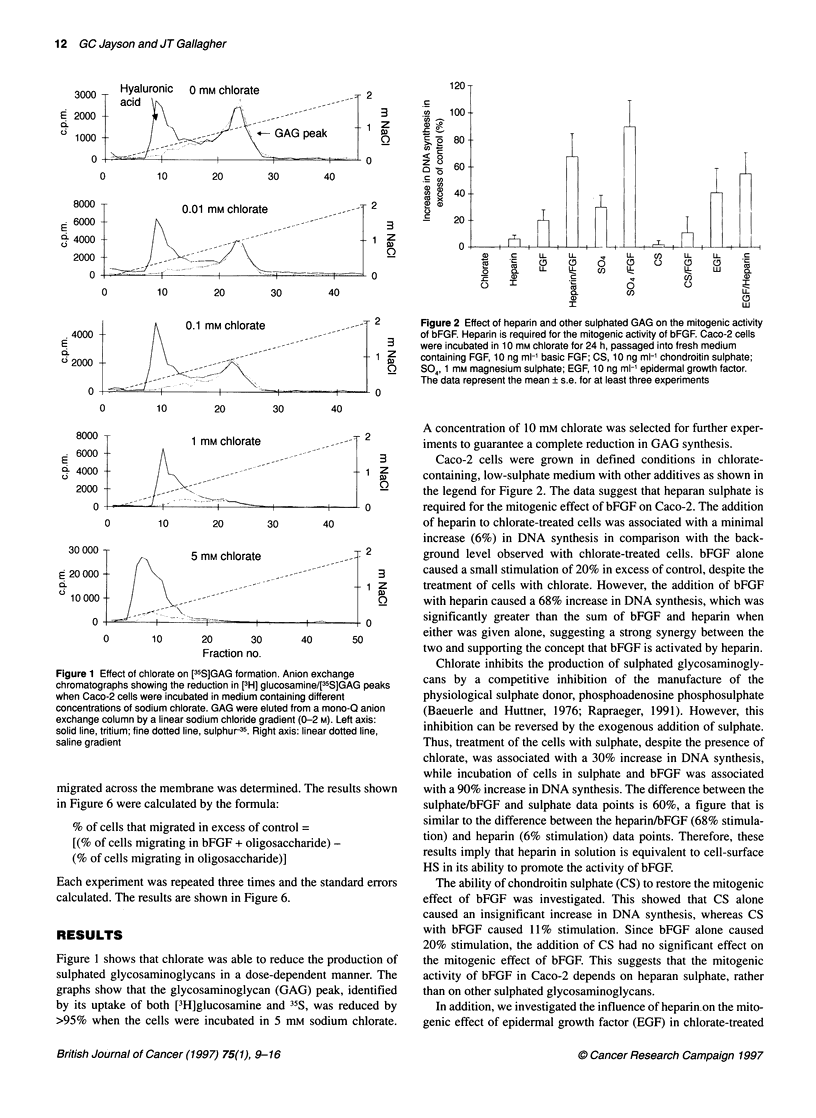
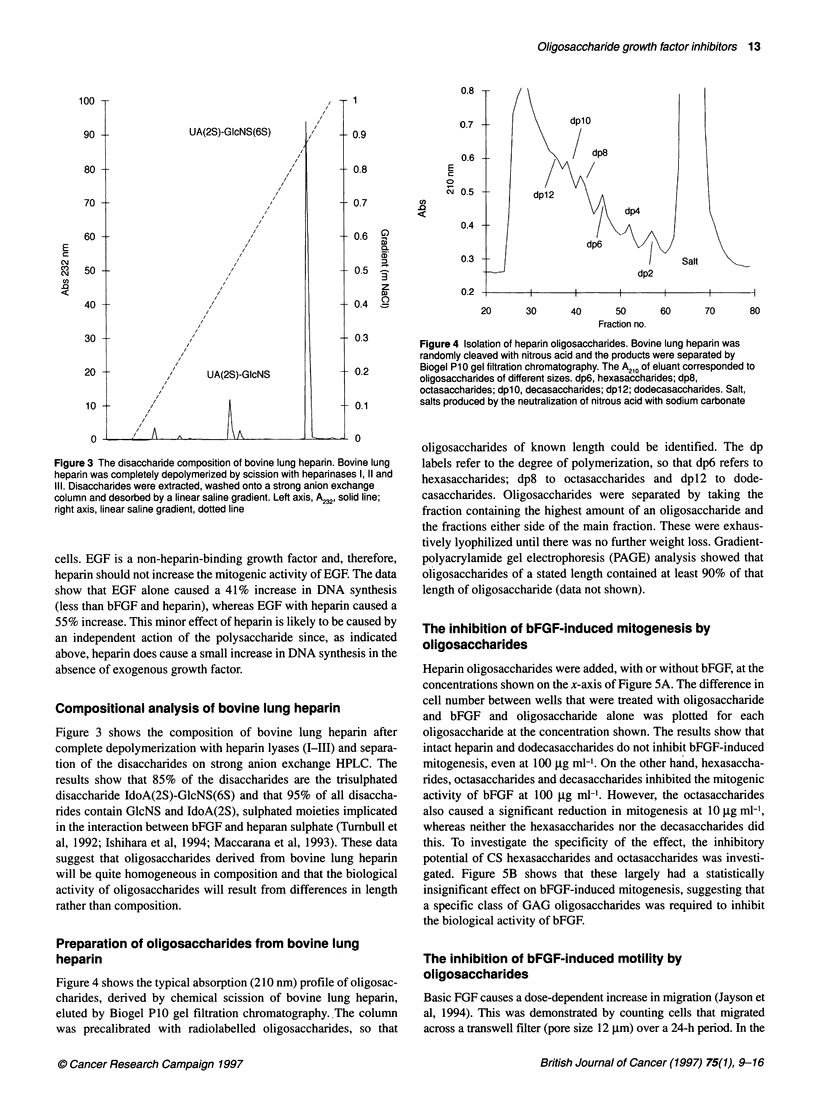
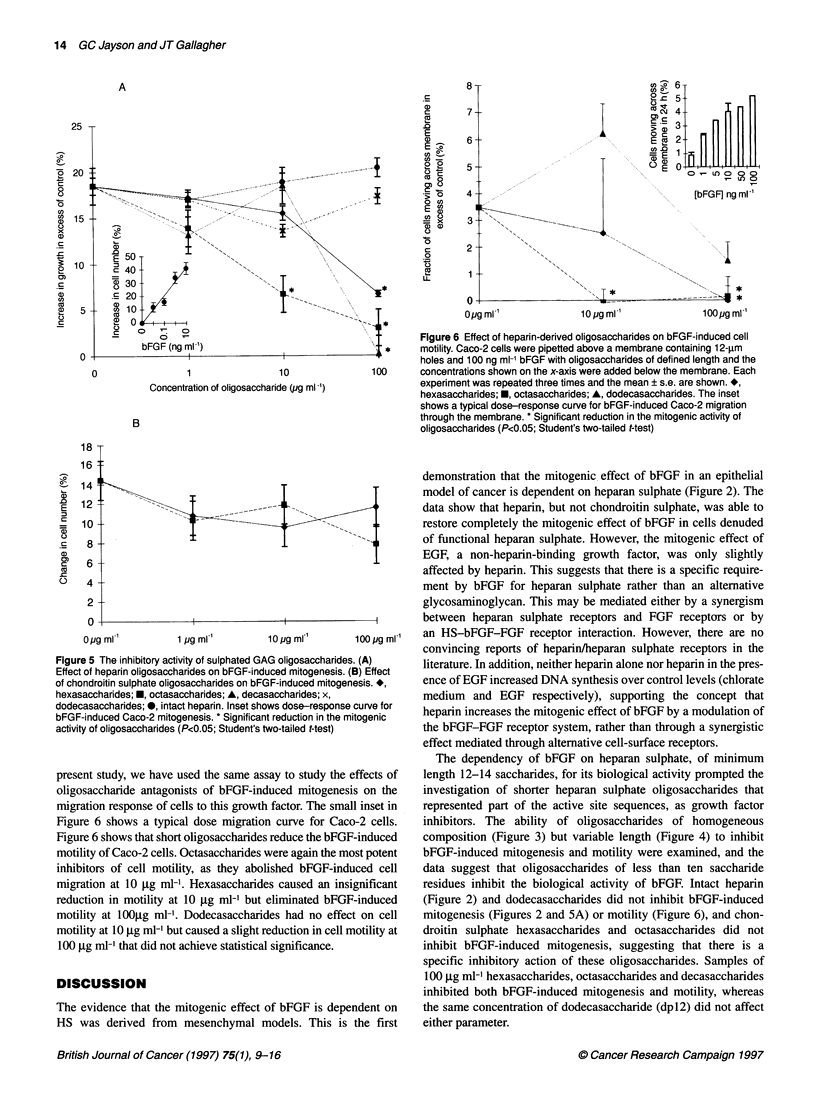
A number of growth factors, including members of the fibroblast growth factor (FGF) family - hepatocyte growth factor, vascular endothelial growth factor and heparin-binding epidermal growth factor - are dependent on heparan sulphate (HS) for biological activity mediated through their high-affinity signal-transducing receptors. This obligate requirement for HS prompted the search for antagonists of HS function that could be used as anti-growth factor drugs for the treatment of cancer. Basic FGF (bFGF) was the focus of this study. Caco-2, a human colon carcinoma cell line, was adapted to growth in serum-free medium so that investigation of its growth factor requirements for growth and migration could be performed in defined conditions (Jayson GC, Evans GS, Pemberton PW, Lobley RW, Allen T 1994, Cancer Res, 54, 5718-5723). This cell line multiplied and moved in a dose-dependent manner in response to bFGF. Here, we show that the mitogenic response to bFGF is dependent on the presence of heparan sulphate. A library of heparin oligosaccharides with uniform composition but variable length was generated [general formula [IdoA(2S)-GlcNS(6S)n], and oligosaccharides of defined lengths were tested for their ability to inhibit the biological activity of bFGF. While intact heparin and heparin-derived fragments of 12 monosaccharide units did not affect bFGF-induced cell division or bFGF-induced cell migration, octasaccharides and decasaccharides potently inhibited the bFGF-induced growth and migration responses. In particular, octasaccharides completely inhibited these biological activities at 10 microg ml-, a clinically achievable and tolerable concentration. This study shows that the length of an oligosaccharide determines its ability to block the biological activity of bFGF. The observation that the biological activity of cell-surface heparan sulphate can be antagonized in this way in a human carcinoma cell line suggests that oligosaccharides should be investigated further as anti-growth factor agents for the treatment of cancer. In addition, the results suggest that the clinical evaluation of low-molecular weight heparin (LMWH) as an anti-cancer agent might benefit from subfractionation of the LMWH, to remove oligosaccharides of 12 or more residues.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviezer D., Levy E., Safran M., Svahn C., Buddecke E., Schmidt A., David G., Vlodavsky I., Yayon A. Differential structural requirements of heparin and heparan sulfate proteoglycans that promote binding of basic fibroblast growth factor to its receptor. J Biol Chem. 1994 Jan 7;269(1):114–121. [PubMed] [Google Scholar]
- Baeuerle P. A., Huttner W. B. Chlorate--a potent inhibitor of protein sulfation in intact cells. Biochem Biophys Res Commun. 1986 Dec 15;141(2):870–877. doi: 10.1016/s0006-291x(86)80253-4. [DOI] [PubMed] [Google Scholar]
- Bara L., Billaud E., Gramond G., Kher A., Samama M. Comparative pharmacokinetics of a low molecular weight heparin (PK 10 169) and unfractionated heparin after intravenous and subcutaneous administration. Thromb Res. 1985 Sep 1;39(5):631–636. doi: 10.1016/0049-3848(85)90244-0. [DOI] [PubMed] [Google Scholar]
- Coltrini D., Rusnati M., Zoppetti G., Oreste P., Grazioli G., Naggi A., Presta M. Different effects of mucosal, bovine lung and chemically modified heparin on selected biological properties of basic fibroblast growth factor. Biochem J. 1994 Oct 15;303(Pt 2):583–590. doi: 10.1042/bj3030583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger M. A., Reyno L. M., Jodrell D. I., Sinibaldi V. J., Tkaczuk K. H., Sridhara R., Zuhowski E. G., Lowitt M. H., Jacobs S. C., Egorin M. J. Suramin, an active drug for prostate cancer: interim observations in a phase I trial. J Natl Cancer Inst. 1993 Apr 21;85(8):611–621. doi: 10.1093/jnci/85.8.611. [DOI] [PubMed] [Google Scholar]
- Gitay-Goren H., Soker S., Vlodavsky I., Neufeld G. The binding of vascular endothelial growth factor to its receptors is dependent on cell surface-associated heparin-like molecules. J Biol Chem. 1992 Mar 25;267(9):6093–6098. [PubMed] [Google Scholar]
- Guimond S., Maccarana M., Olwin B. B., Lindahl U., Rapraeger A. C. Activating and inhibitory heparin sequences for FGF-2 (basic FGF). Distinct requirements for FGF-1, FGF-2, and FGF-4. J Biol Chem. 1993 Nov 15;268(32):23906–23914. [PubMed] [Google Scholar]
- Hahnenberger R., Jakobson A. M., Ansari A., Wehler T., Svahn C. M., Lindahl U. Low-sulphated oligosaccharides derived from heparan sulphate inhibit normal angiogenesis. Glycobiology. 1993 Dec;3(6):567–573. doi: 10.1093/glycob/3.6.567. [DOI] [PubMed] [Google Scholar]
- Higashiyama S., Abraham J. A., Klagsbrun M. Heparin-binding EGF-like growth factor stimulation of smooth muscle cell migration: dependence on interactions with cell surface heparan sulfate. J Cell Biol. 1993 Aug;122(4):933–940. doi: 10.1083/jcb.122.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh J., Levine M. N. Low molecular weight heparin. Blood. 1992 Jan 1;79(1):1–17. [PubMed] [Google Scholar]
- Ishihara M., Shaklee P. N., Yang Z., Liang W., Wei Z., Stack R. J., Holme K. Structural features in heparin which modulate specific biological activities mediated by basic fibroblast growth factor. Glycobiology. 1994 Aug;4(4):451–458. doi: 10.1093/glycob/4.4.451. [DOI] [PubMed] [Google Scholar]
- Ishihara M. Structural requirements in heparin for binding and activation of FGF-1 and FGF-4 are different from that for FGF-2. Glycobiology. 1994 Dec;4(6):817–824. doi: 10.1093/glycob/4.6.817. [DOI] [PubMed] [Google Scholar]
- Ishihara M., Tyrrell D. J., Stauber G. B., Brown S., Cousens L. S., Stack R. J. Preparation of affinity-fractionated, heparin-derived oligosaccharides and their effects on selected biological activities mediated by basic fibroblast growth factor. J Biol Chem. 1993 Mar 5;268(7):4675–4683. [PubMed] [Google Scholar]
- Jayson G. C., Evans G. S., Pemberton P. W., Lobley R. W., Allen T. Basic fibroblast growth factor increases the multiplication and migration of a serum-free derivative of CACO-2 but does not affect differentiation. Cancer Res. 1994 Nov 1;54(21):5718–5723. [PubMed] [Google Scholar]
- Kan M., Wang F., Xu J., Crabb J. W., Hou J., McKeehan W. L. An essential heparin-binding domain in the fibroblast growth factor receptor kinase. Science. 1993 Mar 26;259(5103):1918–1921. doi: 10.1126/science.8456318. [DOI] [PubMed] [Google Scholar]
- Maccarana M., Casu B., Lindahl U. Minimal sequence in heparin/heparan sulfate required for binding of basic fibroblast growth factor. J Biol Chem. 1993 Nov 15;268(32):23898–23905. [PubMed] [Google Scholar]
- Mustonen T., Alitalo K. Endothelial receptor tyrosine kinases involved in angiogenesis. J Cell Biol. 1995 May;129(4):895–898. doi: 10.1083/jcb.129.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers C., Cooper M., Stein C., LaRocca R., Walther M. M., Weiss G., Choyke P., Dawson N., Steinberg S., Uhrich M. M. Suramin: a novel growth factor antagonist with activity in hormone-refractory metastatic prostate cancer. J Clin Oncol. 1992 Jun;10(6):881–889. doi: 10.1200/JCO.1992.10.6.881. [DOI] [PubMed] [Google Scholar]
- Olwin B. B., Rapraeger A. Repression of myogenic differentiation by aFGF, bFGF, and K-FGF is dependent on cellular heparan sulfate. J Cell Biol. 1992 Aug;118(3):631–639. doi: 10.1083/jcb.118.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz D. M., Herr A. B., Nilsson M., Westman J., Svahn C. M., Waksman G. FGF binding and FGF receptor activation by synthetic heparan-derived di- and trisaccharides. Science. 1995 Apr 21;268(5209):432–436. doi: 10.1126/science.7536345. [DOI] [PubMed] [Google Scholar]
- Ornitz D. M., Yayon A., Flanagan J. G., Svahn C. M., Levi E., Leder P. Heparin is required for cell-free binding of basic fibroblast growth factor to a soluble receptor and for mitogenesis in whole cells. Mol Cell Biol. 1992 Jan;12(1):240–247. doi: 10.1128/mcb.12.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestrelski S. J., Fox G. M., Arakawa T. Binding of heparin to basic fibroblast growth factor induces a conformational change. Arch Biochem Biophys. 1992 Mar;293(2):314–319. doi: 10.1016/0003-9861(92)90401-h. [DOI] [PubMed] [Google Scholar]
- Rapraeger A. C., Krufka A., Olwin B. B. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science. 1991 Jun 21;252(5013):1705–1708. doi: 10.1126/science.1646484. [DOI] [PubMed] [Google Scholar]
- Shively J. E., Conrad H. E. Formation of anhydrosugars in the chemical depolymerization of heparin. Biochemistry. 1976 Sep 7;15(18):3932–3942. doi: 10.1021/bi00663a005. [DOI] [PubMed] [Google Scholar]
- Turnbull J. E., Fernig D. G., Ke Y., Wilkinson M. C., Gallagher J. T. Identification of the basic fibroblast growth factor binding sequence in fibroblast heparan sulfate. J Biol Chem. 1992 May 25;267(15):10337–10341. [PubMed] [Google Scholar]
- Turnbull J. E., Gallagher J. T. Distribution of iduronate 2-sulphate residues in heparan sulphate. Evidence for an ordered polymeric structure. Biochem J. 1991 Feb 1;273(Pt 3):553–559. doi: 10.1042/bj2730553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker A., Turnbull J. E., Gallagher J. T. Specific heparan sulfate saccharides mediate the activity of basic fibroblast growth factor. J Biol Chem. 1994 Jan 14;269(2):931–935. [PubMed] [Google Scholar]
- Yayon A., Klagsbrun M., Esko J. D., Leder P., Ornitz D. M. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991 Feb 22;64(4):841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]


