Abstract
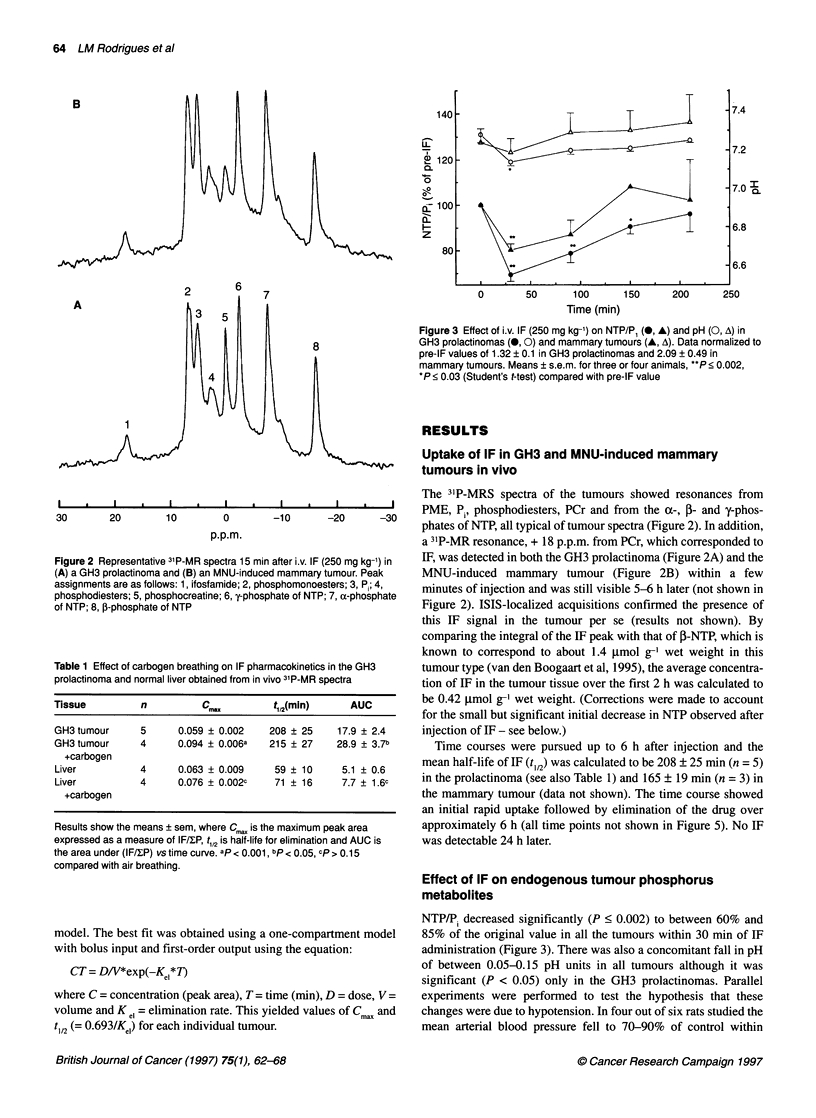
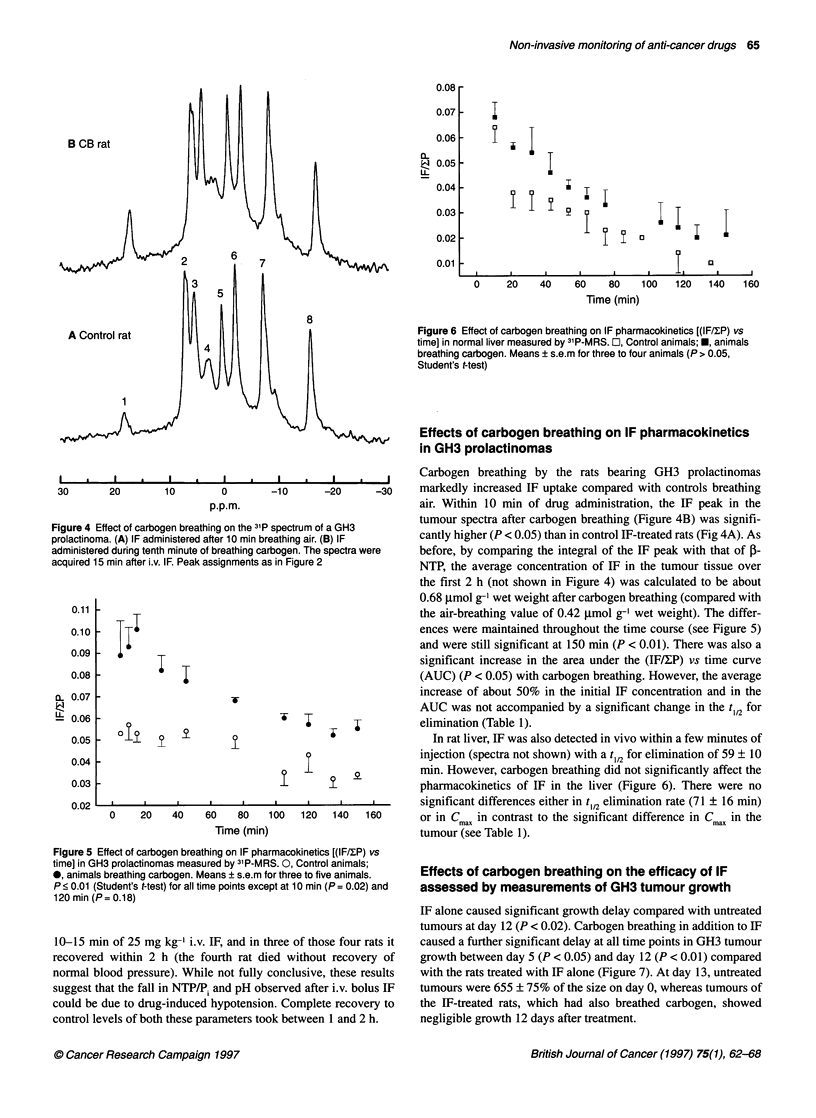
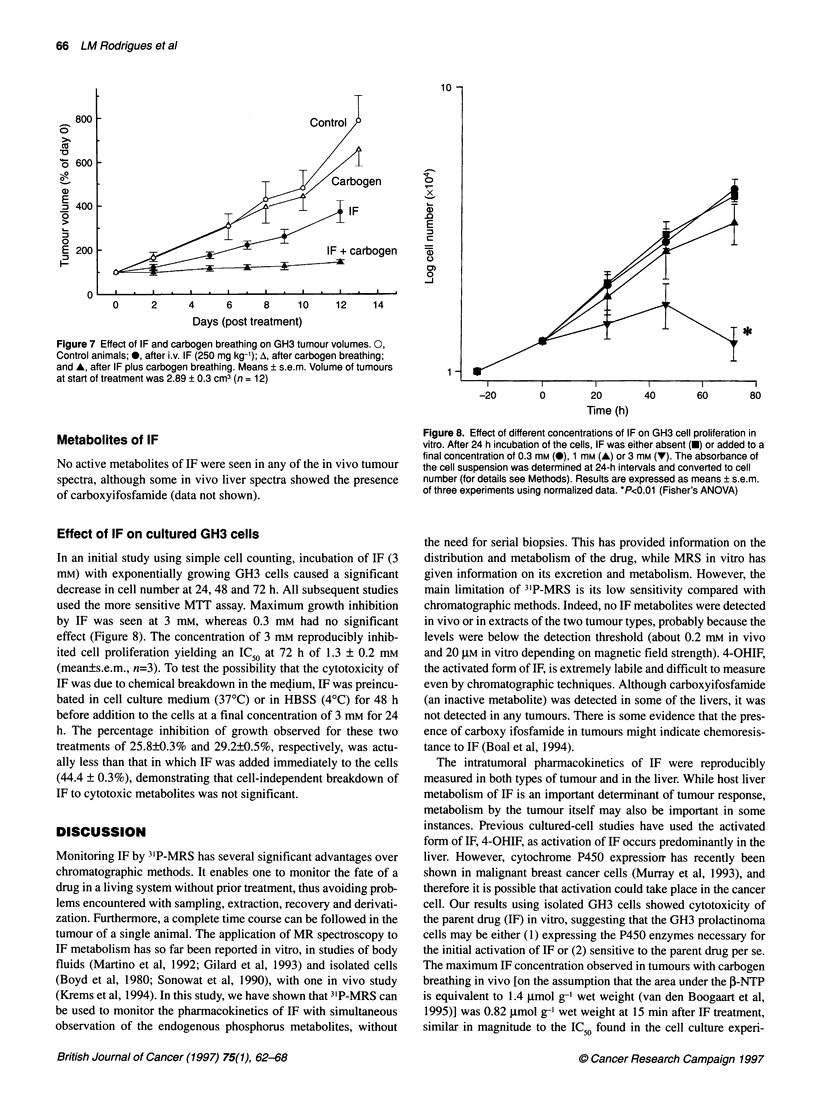
The direct detection and monitoring of anti-cancer drugs in vivo by magnetic resonance spectroscopy (MRS) may lead to improved anti-cancer strategies. 31P-MRS has been used to detect and quantify ifosfamide (IF) in vivo in GH3 prolactinomas and N-methyl-N-nitrosourea (MNU)-induced mammary tumours in rats. The average concentration of IF in the GH3 prolactinoma over the first 2 h following a dose of 250 mg kg-1 i.v. was calculated to be 0.42 micromol g-1 wet weight, with a half-life of elimination (t1/2) of 2-4 h. Carbogen (95% oxygen/5% carbon dioxide) breathing increased the amount of IF taken up by the GH3 prolactinoma by 50% (P<0.01) to 0.68 micromol g-1 wet weight, although t1/2 elimination rates were unchanged. IF was also detected in the liver in vivo, with a t1/2 of about 1 h. Carbogen breathing did not affect the maximum peak area (Cmax) or the t1/2 in the liver. Most importantly, the carbogen-induced increase in IF uptake by the tumour caused significant growth delay at all time points in the GH3 tumour growth between day 5 and day 12 (P< 0.01) compared with IF alone. These findings show that carbogen breathing has potential for increasing the efficacy of anti-cancer drugs. Isolated GH3 cells were sensitive to the parent drug (IF) in vitro (IC50 = 1.3 +/- 0.2 mM) suggesting that the GH3 cells may be either expressing P450 enzymes or are sensitive to the parent drug per se.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen L. M., Creaven P. J., Nelson R. L. Studies on the human pharmacokinetics of isophosphamide (NSC-109724). Cancer Treat Rep. 1976 Apr;60(4):451–458. [PubMed] [Google Scholar]
- Artemov D., Bhujwalla Z. M., Maxwell R. J., Griffiths J. R., Judson I. R., Leach M. O., Glickson J. D. Pharmacokinetics of the 13C labeled anticancer agent temozolomide detected in vivo by selective cross-polarization transfer. Magn Reson Med. 1995 Sep;34(3):338–342. doi: 10.1002/mrm.1910340310. [DOI] [PubMed] [Google Scholar]
- Boal J. H., Ludeman S. M., Ho C. K., Engel J., Niemeyer U. Direct detection of the intracellular formation of carboxyphosphamides using nuclear magnetic resonance spectroscopy. Arzneimittelforschung. 1994 Jan;44(1):84–93. [PubMed] [Google Scholar]
- Boddy A. V., Yule S. M., Wyllie R., Price L., Pearson A. D., Idle J. R. Pharmacokinetics and metabolism of ifosfamide administered as a continuous infusion in children. Cancer Res. 1993 Aug 15;53(16):3758–3764. [PubMed] [Google Scholar]
- Boyd V. L., Robbins J. D., Egan W., Ludeman S. M. 31P nuclear magnetic resonance spectroscopic observation of the intracellular transformations of oncostatic cyclophosphamide metabolites. J Med Chem. 1986 Jul;29(7):1206–1210. doi: 10.1021/jm00157a015. [DOI] [PubMed] [Google Scholar]
- Engle T. W., Zon G., Egan W. 31P NMR investigations of phosphoramide mustard: evaluation of pH control over the rate of intramolecular cyclization to an aziridinium ion and the hydrolysis of this reactive alkylator. J Med Chem. 1979 Aug;22(8):897–899. doi: 10.1021/jm00194a001. [DOI] [PubMed] [Google Scholar]
- Falk S. J., Ward R., Bleehen N. M. The influence of carbogen breathing on tumour tissue oxygenation in man evaluated by computerised p02 histography. Br J Cancer. 1992 Nov;66(5):919–924. doi: 10.1038/bjc.1992.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilard V., Malet-Martino M. C., de Forni M., Niemeyer U., Ader J. C., Martino R. Determination of the urinary excretion of ifosfamide and its phosphorated metabolites by phosphorus-31 nuclear magnetic resonance spectroscopy. Cancer Chemother Pharmacol. 1993;31(5):387–394. doi: 10.1007/BF00686153. [DOI] [PubMed] [Google Scholar]
- Goren M. P., Wright R. K., Pratt C. B., Pell F. E. Dechloroethylation of ifosfamide and neurotoxicity. Lancet. 1986 Nov 22;2(8517):1219–1220. doi: 10.1016/s0140-6736(86)92227-0. [DOI] [PubMed] [Google Scholar]
- He Q., Bhujwalla Z. M., Maxwell R. J., Griffiths J. R., Glickson J. D. Proton NMR observation of the antineoplastic agent Iproplatin in vivo by selective multiple quantum coherence transfer (Sel-MQC). Magn Reson Med. 1995 Mar;33(3):414–416. doi: 10.1002/mrm.1910330315. [DOI] [PubMed] [Google Scholar]
- Honess D. J., Bleehen N. M. Perfusion changes in the RIF-1 tumour and normal tissues after carbogen and nicotinamide, individually and combined. Br J Cancer. 1995 Jun;71(6):1175–1180. doi: 10.1038/bjc.1995.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issels R. D., Prenninger S. W., Nagele A., Boehm E., Sauer H., Jauch K. W., Denecke H., Berger H., Peter K., Wilmanns W. Ifosfamide plus etoposide combined with regional hyperthermia in patients with locally advanced sarcomas: a phase II study. J Clin Oncol. 1990 Nov;8(11):1818–1829. doi: 10.1200/JCO.1990.8.11.1818. [DOI] [PubMed] [Google Scholar]
- Kaijser G. P., Beijnen J. H., Bult A., Underberg W. J. Ifosfamide metabolism and pharmacokinetics (review). Anticancer Res. 1994 Mar-Apr;14(2A):517–531. [PubMed] [Google Scholar]
- Lind M. J., Margison J. M., Cerny T., Thatcher N., Wilkinson P. M. Comparative pharmacokinetics and alkylating activity of fractionated intravenous and oral ifosfamide in patients with bronchogenic carcinoma. Cancer Res. 1989 Feb 1;49(3):753–757. [PubMed] [Google Scholar]
- Malet-Martino M. C., Martino R. Magnetic resonance spectroscopy: a powerful tool for drug metabolism studies. Biochimie. 1992 Sep-Oct;74(9-10):785–800. doi: 10.1016/0300-9084(92)90061-i. [DOI] [PubMed] [Google Scholar]
- Martino R., Crasnier F., Chouini-Lalanne N., Gilard V., Niemeyer U., De Forni M., Malet-Martino M. C. A new approach to the study of ifosfamide metabolism by the analysis of human body fluids with 31P nuclear magnetic resonance spectroscopy. J Pharmacol Exp Ther. 1992 Mar;260(3):1133–1144. [PubMed] [Google Scholar]
- Prichard J. W., Alger J. R., Behar K. L., Petroff O. A., Shulman R. G. Cerebral metabolic studies in vivo by 31P NMR. Proc Natl Acad Sci U S A. 1983 May;80(9):2748–2751. doi: 10.1073/pnas.80.9.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prysor-Jones R. A., Jenkins J. S. Effect of bromocriptine on DNA synthesis, growth and hormone secretion of spontaneous pituitary tumours in the rat. J Endocrinol. 1981 Mar;88(3):463–469. doi: 10.1677/joe.0.0880463. [DOI] [PubMed] [Google Scholar]
- Skinner R., Pearson A. D., Price L., Coulthard M. G., Craft A. W. Nephrotoxicity after ifosfamide. Arch Dis Child. 1990 Jul;65(7):732–738. doi: 10.1136/adc.65.7.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek N. E. Metabolism of oxazaphosphorines. Pharmacol Ther. 1988;37(3):301–355. doi: 10.1016/0163-7258(88)90004-6. [DOI] [PubMed] [Google Scholar]
- Sonawat H. M., Leibfritz D., Engel J., Hilgard P. Biotransformation of mafosfamide in P388 mice leukemia cells: intracellular 31P-NMR studies. Biochim Biophys Acta. 1990 Apr 9;1052(1):36–41. doi: 10.1016/0167-4889(90)90054-h. [DOI] [PubMed] [Google Scholar]
- Vaupel P., Kallinowski F., Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989 Dec 1;49(23):6449–6465. [PubMed] [Google Scholar]
- Wagner T., Heydrich D., Jork T., Voelcker G., Hohorst H. J. Comparative study on human pharmacokinetics of activated ifosfamide and cyclophosphamide by a modified fluorometric test. J Cancer Res Clin Oncol. 1981;100(1):95–104. doi: 10.1007/BF00405906. [DOI] [PubMed] [Google Scholar]
- Wiedemann G. J., Siemens H. J., Mentzel M., Biersack A., Wössmann W., Knocks D., Weiss C., Wagner T. Effects of temperature on the therapeutic efficacy and pharmacokinetics of ifosfamide. Cancer Res. 1993 Sep 15;53(18):4268–4272. [PubMed] [Google Scholar]
- Williams J. C., Gusterson B. A., Monaghan P., Coombes R. C., Rudland P. S. Isolation and characterization of clonal cell lines from a transplantable metastasizing rat mammary tumor, TR2CL. J Natl Cancer Inst. 1985 Feb;74(2):415–428. [PubMed] [Google Scholar]
- van den Boogaart A., Howe F. A., Rodrigues L. M., Stubbs M., Griffiths J. R. In vivo 31P MRS: absolute concentrations, signal-to-noise and prior knowledge. NMR Biomed. 1995 Apr;8(2):87–93. doi: 10.1002/nbm.1940080207. [DOI] [PubMed] [Google Scholar]
- van der Veen J. W., de Beer R., Luyten P. R., van Ormondt D. Accurate quantification of in vivo 31P NMR signals using the variable projection method and prior knowledge. Magn Reson Med. 1988 Jan;6(1):92–98. doi: 10.1002/mrm.1910060111. [DOI] [PubMed] [Google Scholar]


