Abstract
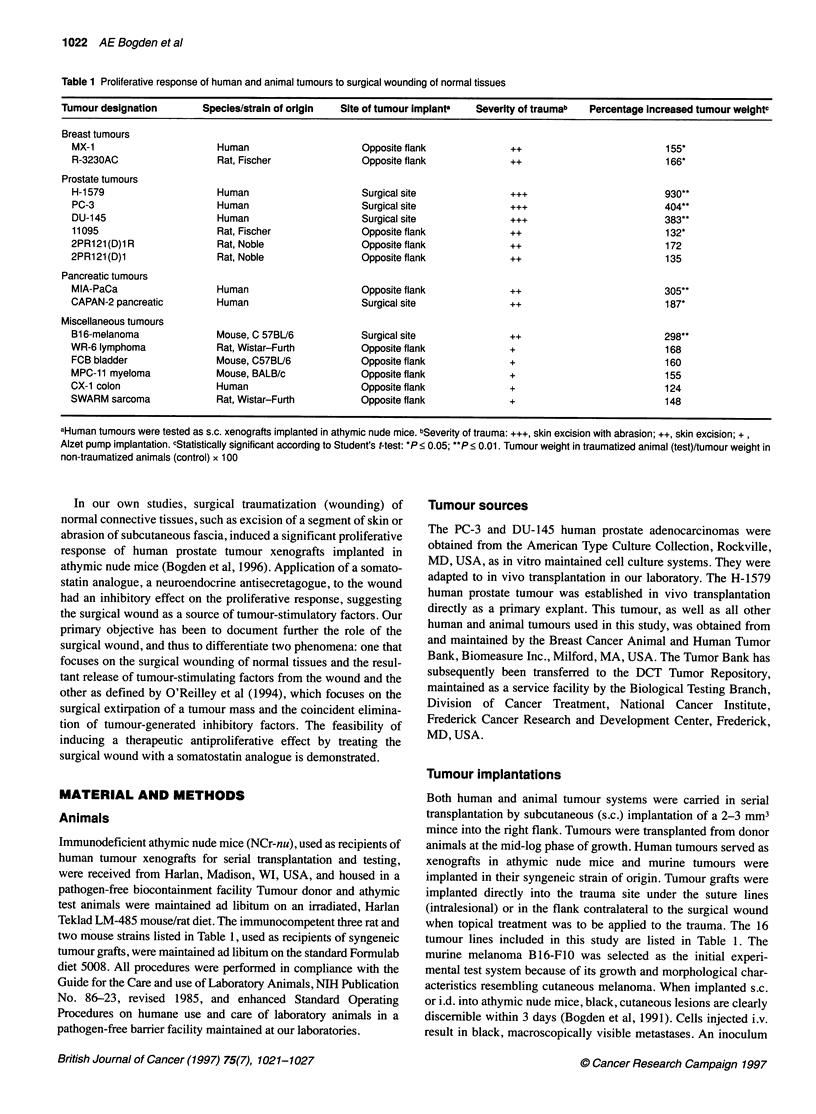
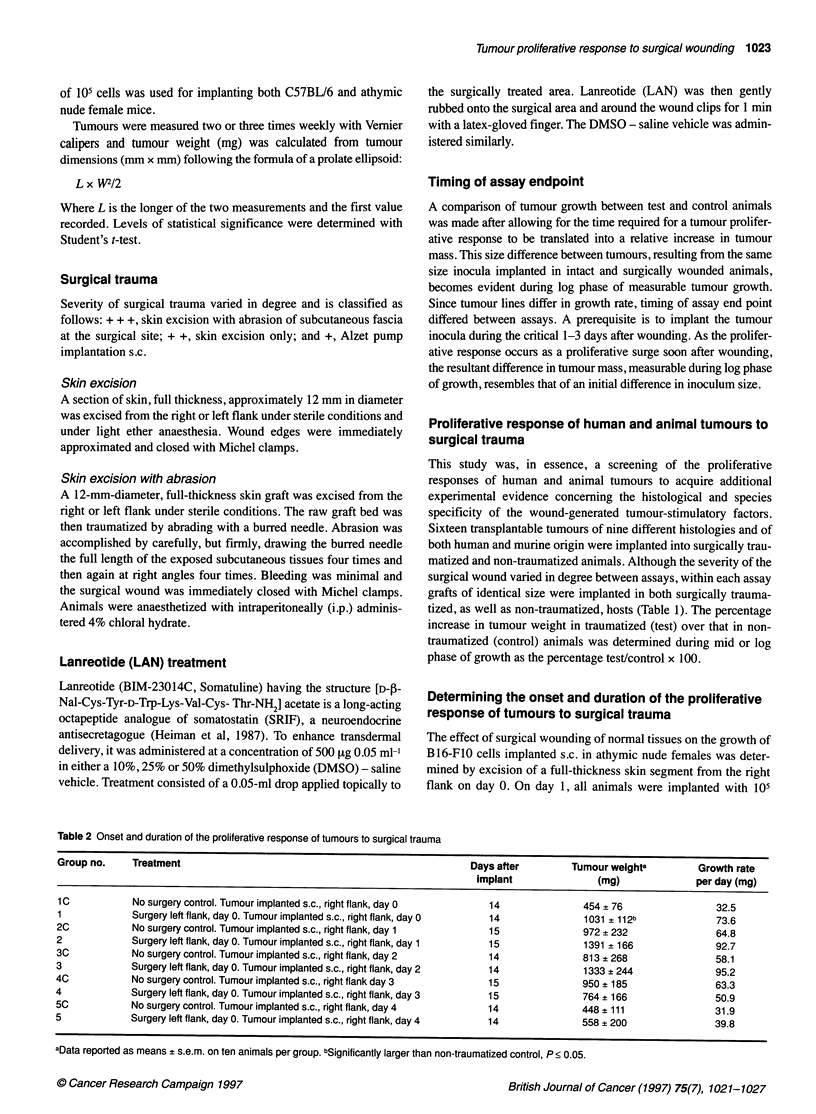
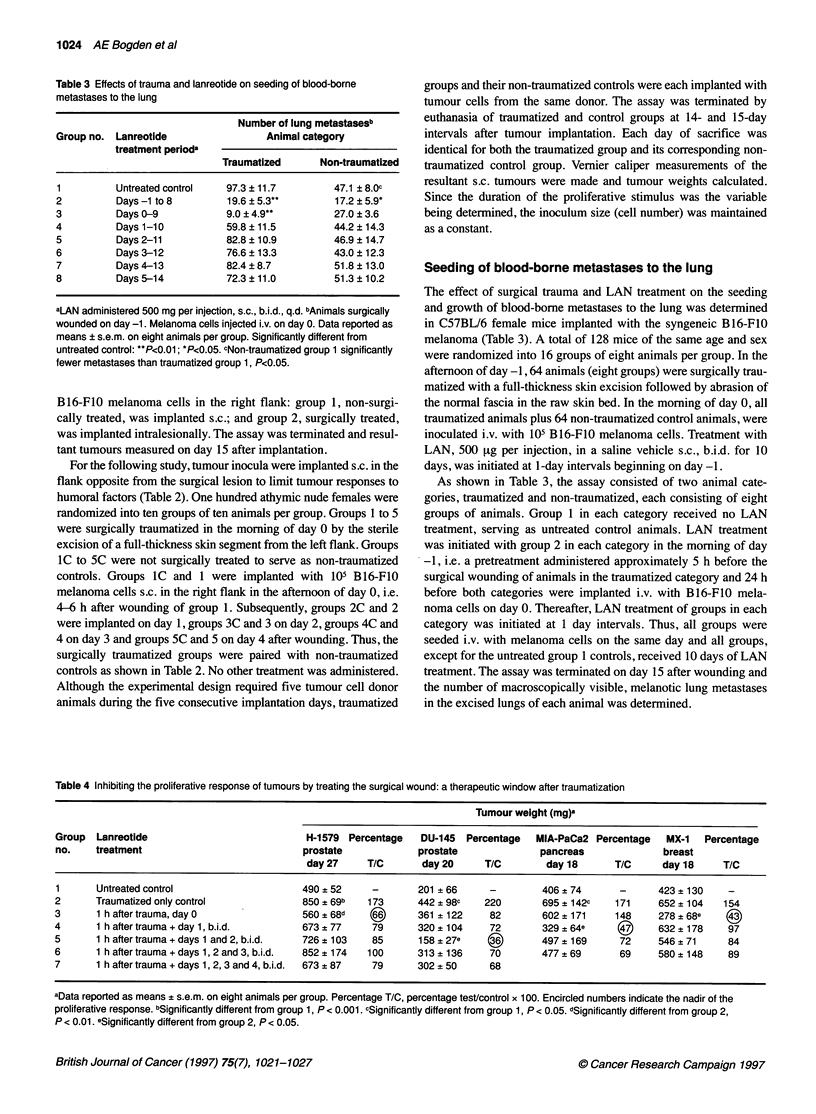
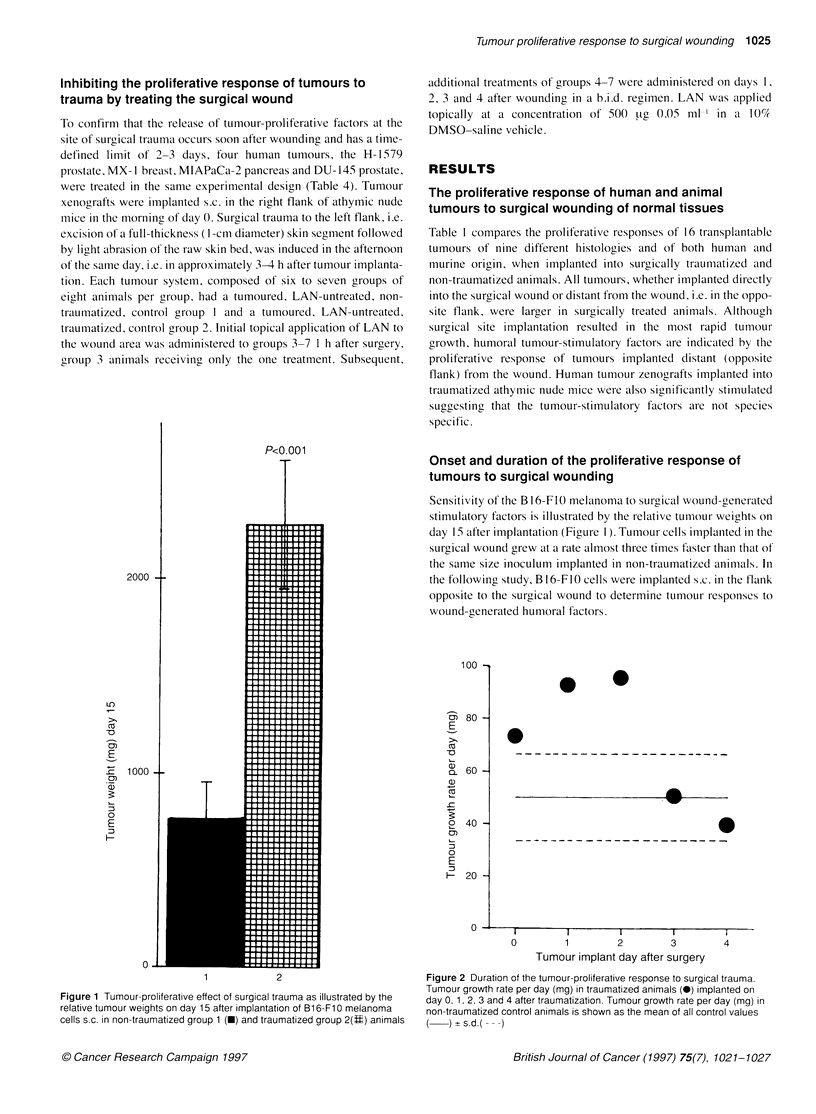
Acceleration of secondary tumour growth and metastases following excision of a primary tumour has been attributed to the consequent removal of primary tumour-generated inhibitory factors. However, our studies have shown that surgical wounding of normal tissues significantly stimulated the growth of malignant tissues without the concomitant presence or excision of a tumour mass. A humoral stimulating component was indicated by the proliferative response of tumours and metastases distant from the surgical wound. All 16 human and murine tumours, of nine different histologies, showed a measurable acceleration of growth when implanted in surgically treated animals, suggesting that the ability of malignant tissue to respond to surgical wounding of normal tissue was not histologically or species specific. The proliferative surge of malignant tissues was detectable soon after wounding and had a duration of 2-3 days. The surgical wound as the source of the tumour-stimulating factor(s) was affirmed by the significant inhibition of tumour proliferative responses when a somatostatin analogue was applied topically to the surgical wound within 1 h of wounding, and/or during the critical tumour-stimulatory period of 1-2 days after wounding. A potential therapeutic window for reducing a risk factor that may be inadvertently imposed upon every surgical/oncology patient is indicated.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amento E. P., Beck L. S. TGF-beta and wound healing. Ciba Found Symp. 1991;157:115–129. doi: 10.1002/9780470514061.ch8. [DOI] [PubMed] [Google Scholar]
- Bennett N. T., Schultz G. S. Growth factors and wound healing: biochemical properties of growth factors and their receptors. Am J Surg. 1993 Jun;165(6):728–737. doi: 10.1016/s0002-9610(05)80797-4. [DOI] [PubMed] [Google Scholar]
- Bogden A. E., LePage D., Zwicker S., Grant W., Silver M. Proliferative response of human prostate tumour xenografts to surgical trauma and the transurethral resection of the prostate controversy. Br J Cancer. 1996 Jan;73(1):73–78. doi: 10.1038/bjc.1996.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiman M. L., Murphy W. A., Coy D. H. Differential binding of somatostatin agonists to somatostatin receptors in brain and adenohypophysis. Neuroendocrinology. 1987 Jun;45(6):429–436. doi: 10.1159/000124788. [DOI] [PubMed] [Google Scholar]
- Kassabian V. S., Bottles K., Weaver R., Williams R. D., Paulson D. F., Scardino P. T. Possible mechanism for seeding of tumor during radical prostatectomy. J Urol. 1993 Oct;150(4):1169–1171. doi: 10.1016/s0022-5347(17)35716-6. [DOI] [PubMed] [Google Scholar]
- Keller R. Elicitation of macroscopic metastases via surgery: various forms of surgical intervention differ in their induction of metastatic outgrowth. Invasion Metastasis. 1983;3(3):183–192. [PubMed] [Google Scholar]
- Lynch S. E., Colvin R. B., Antoniades H. N. Growth factors in wound healing. Single and synergistic effects on partial thickness porcine skin wounds. J Clin Invest. 1989 Aug;84(2):640–646. doi: 10.1172/JCI114210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath M. H. Peptide growth factors and wound healing. Clin Plast Surg. 1990 Jul;17(3):421–432. [PubMed] [Google Scholar]
- Mustoe T. A., Pierce G. F., Thomason A., Gramates P., Sporn M. B., Deuel T. F. Accelerated healing of incisional wounds in rats induced by transforming growth factor-beta. Science. 1987 Sep 11;237(4820):1333–1336. doi: 10.1126/science.2442813. [DOI] [PubMed] [Google Scholar]
- O'Reilly M. S., Holmgren L., Shing Y., Chen C., Rosenthal R. A., Moses M., Lane W. S., Cao Y., Sage E. H., Folkman J. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994 Oct 21;79(2):315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- Turnbull R. B., Jr, Kyle K., Watson F. R., Spratt J. Cancer of the colon: the influence of the no-touch isolation technic on survival rates. Ann Surg. 1967 Sep;166(3):420–427. doi: 10.1097/00000658-196709000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyzzer E. E. Factors in the Production and Growth of tumor Metastases. J Med Res. 1913 Jul;28(2):309–332.1. [PMC free article] [PubMed] [Google Scholar]


