Abstract
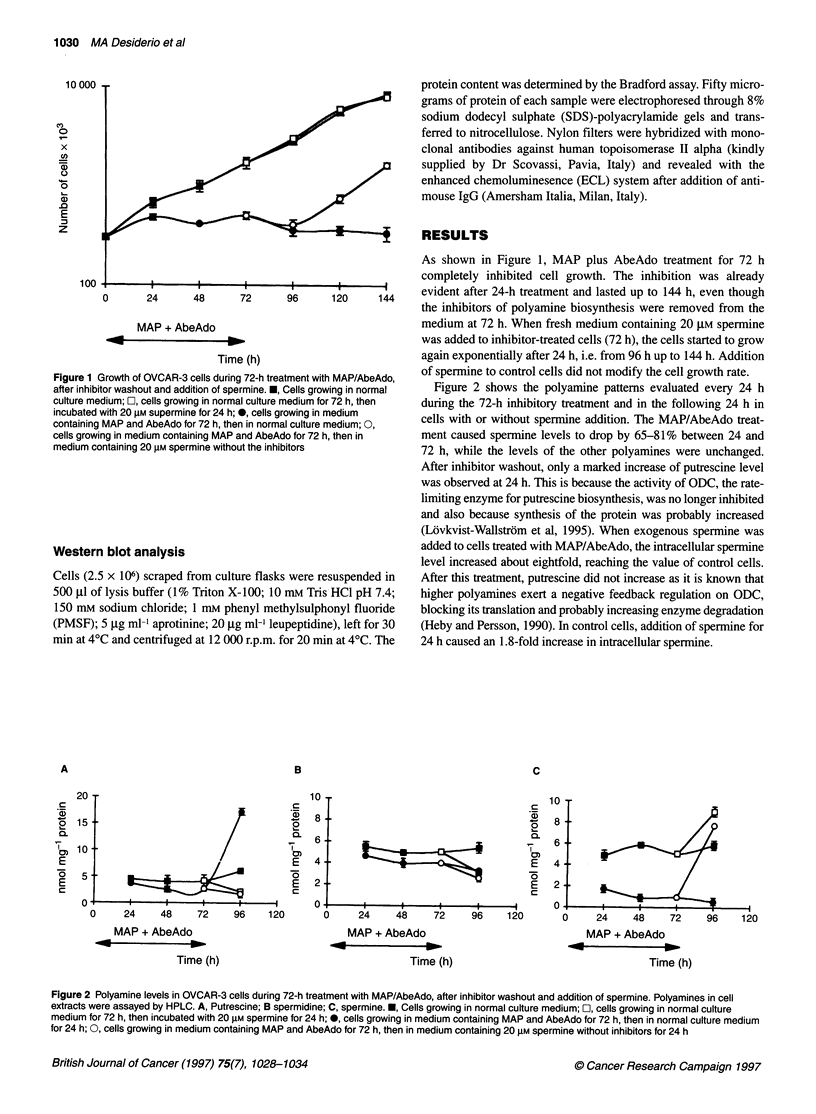
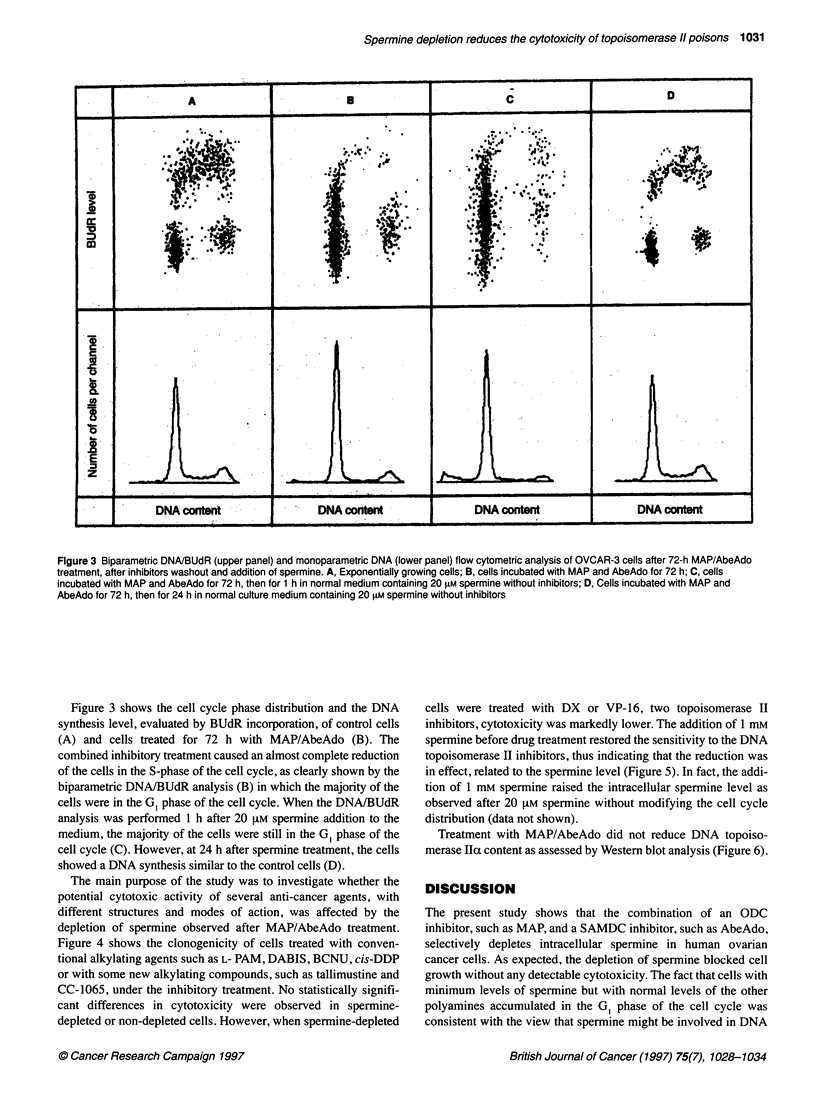
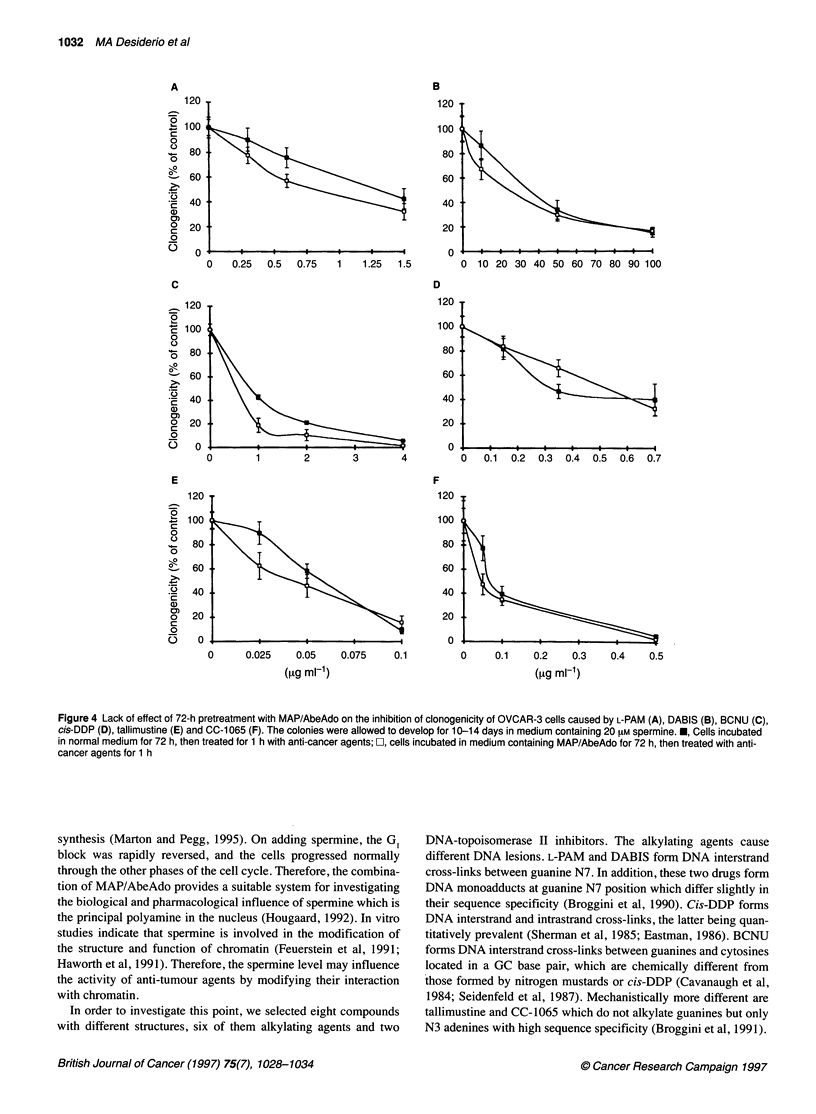
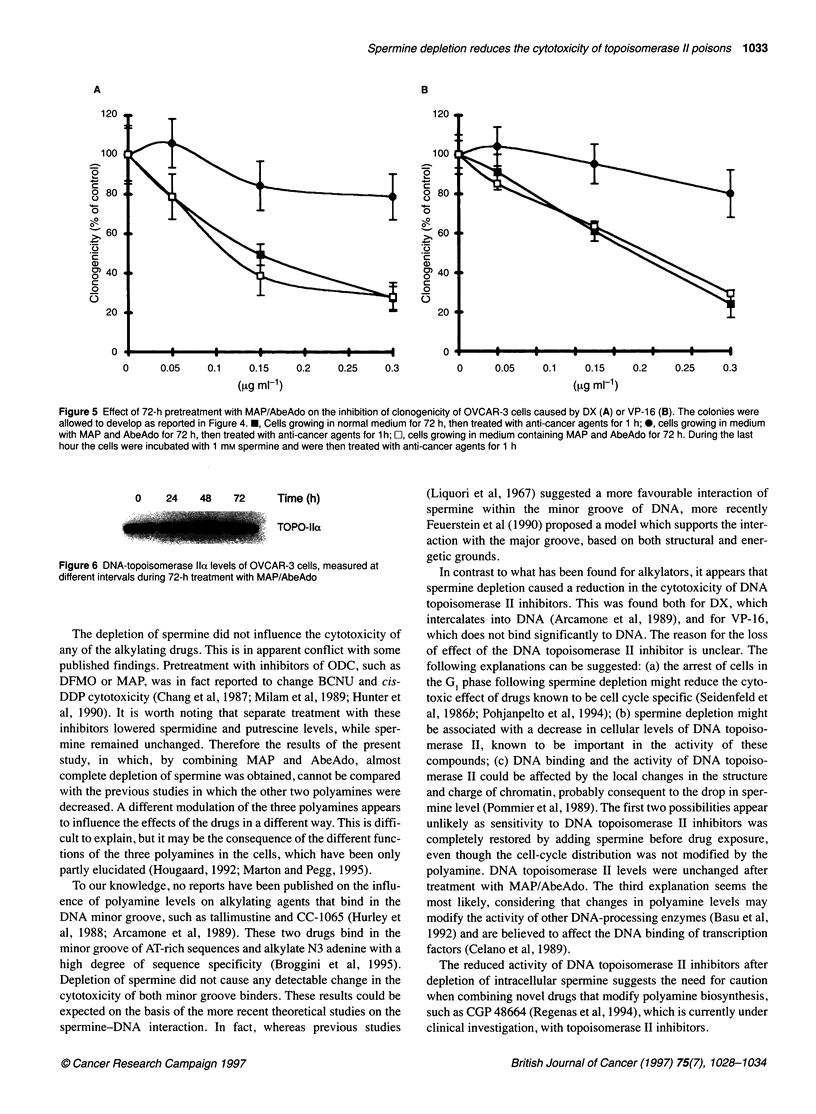
Inhibitors of ornithine decarboxylase (ODC), such as alpha-difluoromethylornithine (DFMO), may influence the cytotoxicity of anti-tumour agents that interact with DNA. Intracellular levels of putrescine and spermidine were markedly reduced by ODC inhibitors while the level of spermine, which is the main polyamine in nuclei, was unchanged. By combining a novel inhibitor of ODC, such as (2R, 5R)-6-heptyne-2,5-diamine (MDL 72.175, MAP), with an inhibitor of S-adenosylmethionine decarboxylase (SAMDC), such as 5'-[[(Z)-4-aminobut-2-enyl]methylamino]-5'-deoxyadenosine (MDL 73.811, AbeAdo), spermine was selectively depleted in a human ovarian cancer cell line OVCAR-3 (i.e. spermine became almost undetectable whereas the levels of spermidine and putrescine were not affected). The depletion of spermine blocked DNA synthesis with a consequent accumulation of cells in the G1 phase of the cell cycle. Pretreatment with MAP plus AbeAdo did not change the cytotoxicity of alkylating agents, such as L-phenylalanine mustard (L-PAM), 1,4-bis(2'-chloroethyl)-1,4-diazabicyclo-[2.2.1] heptane diperchlorate (DABIS), 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU), cis-diamminedichloroplatinum (II) (cis-DDP), N-deformyl-N-[4-N-N,N-bis (2-chloroethylamino)benzoyl] (tallimustine) or CC-1065, whereas it markedly reduced the cytotoxicity of DNA topoisomerase II inhibitors, such as doxorubicin (DX) and 4'-demethylepipodophyllotoxin-5-(4,6-O)-ethylidene- beta-D-glycopyranoside (VP-16). The addition of spermine before drug treatment restored the sensitivity to the DNA topoisomerase II inhibitors, thus indicating that the reduced effect was related to the intracellular spermine level. The reason for the reduction in cytotoxicity is unclear, but it does not appear to be related to a cell cycle effect or to a decrease in the intracellular level of DNA topoisomerase II. Drugs that modify polyamine biosynthesis are under early clinical development as potential new anti-tumour agents. These findings illustrate the need for caution in combining such drugs with DNA topoisomerase II inhibitors.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arcamone F. M., Animati F., Barbieri B., Configliacchi E., D'Alessio R., Geroni C., Giuliani F. C., Lazzari E., Menozzi M., Mongelli N. Synthesis, DNA-binding properties, and antitumor activity of novel distamycin derivatives. J Med Chem. 1989 Apr;32(4):774–778. doi: 10.1021/jm00124a008. [DOI] [PubMed] [Google Scholar]
- Basu H. S., Sturkenboom M. C., Delcros J. G., Csokan P. P., Szollosi J., Feuerstein B. G., Marton L. J. Effect of polyamine depletion on chromatin structure in U-87 MG human brain tumour cells. Biochem J. 1992 Mar 15;282(Pt 3):723–727. doi: 10.1042/bj2820723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broggini M., Coley H. M., Mongelli N., Pesenti E., Wyatt M. D., Hartley J. A., D'Incalci M. DNA sequence-specific adenine alkylation by the novel antitumor drug tallimustine (FCE 24517), a benzoyl nitrogen mustard derivative of distamycin. Nucleic Acids Res. 1995 Jan 11;23(1):81–87. doi: 10.1093/nar/23.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broggini M., Erba E., Ponti M., Ballinari D., Geroni C., Spreafico F., D'Incalci M. Selective DNA interaction of the novel distamycin derivative FCE 24517. Cancer Res. 1991 Jan 1;51(1):199–204. [PubMed] [Google Scholar]
- Broggini M., Hartley J. A., Mattes W. B., Ponti M., Kohn K. W., D'Incalci M. DNA damage and sequence specificity of DNA binding of the new anti-cancer agent 1,4-bis(2'-chloroethyl)-1,4-diazabicyclo-[2.2.1] heptane dimaleate (Dabis maleate) Br J Cancer. 1990 Feb;61(2):285–289. doi: 10.1038/bjc.1990.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh P. F., Jr, Pavelic Z. P., Porter C. W. Enhancement of 1,3-bis(2-chloroethyl)-1-nitrosourea-induced cytotoxicity and DNA damage by alpha-difluoromethylornithine in L1210 leukemia cells. Cancer Res. 1984 Sep;44(9):3856–3861. [PubMed] [Google Scholar]
- Celano P., Baylin S. B., Casero R. A., Jr Polyamines differentially modulate the transcription of growth-associated genes in human colon carcinoma cells. J Biol Chem. 1989 May 25;264(15):8922–8927. [PubMed] [Google Scholar]
- Chang B. K., Gutman R., Chou T. C. Schedule-dependent interaction of alpha-difluoromethylornithine and cis-diamminedichloroplatinum(II) against human and hamster pancreatic cancer cell lines. Cancer Res. 1987 May 1;47(9):2247–2250. [PubMed] [Google Scholar]
- Danzin C., Marchal P., Casara P. Irreversible inhibition of rat S-adenosylmethionine decarboxylase by 5'-([(Z)-4-amino-2-butenyl]methylamino)-5'-deoxyadenosine. Biochem Pharmacol. 1990 Oct 1;40(7):1499–1503. doi: 10.1016/0006-2952(90)90446-r. [DOI] [PubMed] [Google Scholar]
- Desiderio M. A., Mattei S., Biondi G., Colombo M. P. Cytosolic and nuclear spermidine acetyltransferases in growing NIH 3T3 fibroblasts stimulated with serum or polyamines: relationship to polyamine-biosynthetic decarboxylases and histone acetyltransferase. Biochem J. 1993 Jul 15;293(Pt 2):475–479. doi: 10.1042/bj2930475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desiderio M. A. Opposite responses of nuclear spermidine N8-acetyltransferase and histone acetyltransferase activities to regenerative stimuli in rat liver. Hepatology. 1992 May;15(5):928–933. doi: 10.1002/hep.1840150529. [DOI] [PubMed] [Google Scholar]
- Eastman A. Reevaluation of interaction of cis-dichloro(ethylenediamine)platinum(II) with DNA. Biochemistry. 1986 Jul 1;25(13):3912–3915. doi: 10.1021/bi00361a026. [DOI] [PubMed] [Google Scholar]
- Erba E., Mascellani E., Pifferi A., D'Incalci M. Comparison of cell-cycle phase perturbations induced by the DNA-minor-groove alkylator tallimustine and by melphalan in the SW626 cell line. Int J Cancer. 1995 Jul 17;62(2):170–175. doi: 10.1002/ijc.2910620211. [DOI] [PubMed] [Google Scholar]
- Feuerstein B. G., Pattabiraman N., Marton L. J. Molecular mechanics of the interactions of spermine with DNA: DNA bending as a result of ligand binding. Nucleic Acids Res. 1990 Mar 11;18(5):1271–1282. doi: 10.1093/nar/18.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerstein B. G., Williams L. D., Basu H. S., Marton L. J. Implications and concepts of polyamine-nucleic acid interactions. J Cell Biochem. 1991 May;46(1):37–47. doi: 10.1002/jcb.240460107. [DOI] [PubMed] [Google Scholar]
- Haworth I. S., Rodger A., Richards W. G. A molecular mechanics study of spermine complexation to DNA: a new model for spermine-poly(dG-dC) binding. Proc Biol Sci. 1991 May 22;244(1310):107–116. doi: 10.1098/rspb.1991.0058. [DOI] [PubMed] [Google Scholar]
- Heby O., Persson L. Molecular genetics of polyamine synthesis in eukaryotic cells. Trends Biochem Sci. 1990 Apr;15(4):153–158. doi: 10.1016/0968-0004(90)90216-x. [DOI] [PubMed] [Google Scholar]
- Hougaard D. M. Polyamine cytochemistry: localization and possible functions of polyamines. Int Rev Cytol. 1992;138:51–88. doi: 10.1016/s0074-7696(08)61587-9. [DOI] [PubMed] [Google Scholar]
- Hunter K. J., Deen D. F., Pellarin M., Marton L. J. Effect of alpha-difluoromethylornithine on 1,3-bis(2-chloroethyl)-1-nitrosourea and cis-diamminedichloroplatinum(II) cytotoxicity, DNA interstrand cross-linking, and growth in human brain tumor cell lines in vitro. Cancer Res. 1990 May 1;50(9):2769–2772. [PubMed] [Google Scholar]
- Hurley L. H., Lee C. S., McGovren J. P., Warpehoski M. A., Mitchell M. A., Kelly R. C., Aristoff P. A. Molecular basis for sequence-specific DNA alkylation by CC-1065. Biochemistry. 1988 May 17;27(10):3886–3892. doi: 10.1021/bi00410a054. [DOI] [PubMed] [Google Scholar]
- Kamińska B., Kaczmarek L., Grzelakowska-Sztabert B. The regulation of G0-S transition in mouse T lymphocytes by polyamines. Exp Cell Res. 1990 Dec;191(2):239–245. doi: 10.1016/0014-4827(90)90010-8. [DOI] [PubMed] [Google Scholar]
- Krishan A., Frei E., 3rd Effect of adriamycin on the cell cycle traverse and kinetics of cultured human lymphoblasts. Cancer Res. 1976 Jan;36(1):143–150. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Löser C., Wunderlich U., Fölsch U. R. Reversed-phase liquid chromatographic separation and simultaneous fluorimetric detection of polyamines and their monoacetyl derivatives in human and animal urine, serum and tissue samples: an improved, rapid and sensitive method for routine application. J Chromatogr. 1988 Sep 9;430(2):249–262. doi: 10.1016/s0378-4347(00)83160-6. [DOI] [PubMed] [Google Scholar]
- Lövkvist-Wallström E., Stjernborg-Ulvsbäck L., Scheffler I. E., Persson L. Regulation of mammalian ornithine decarboxylase. Studies on the induction of the enzyme by hypotonic stress. Eur J Biochem. 1995 Jul 1;231(1):40–44. [PubMed] [Google Scholar]
- Mach M., Ebert P., Popp R., Ogilvie A. Compartmentalization of polyamines in mammalian cells. Biochem Biophys Res Commun. 1982 Feb 26;104(4):1327–1334. doi: 10.1016/0006-291x(82)91395-x. [DOI] [PubMed] [Google Scholar]
- Mamont P. S., Siat M., Joder-Ohlenbusch A. M., Bernhardt A., Casara P. Effects of (2R, 5R)-6-heptyne-2,5-diamine, a potent inhibitor of L-ornithine decarboxylase, on rat hepatoma cells cultured in vitro. Eur J Biochem. 1984 Aug 1;142(3):457–463. doi: 10.1111/j.1432-1033.1984.tb08308.x. [DOI] [PubMed] [Google Scholar]
- Marton L. J., Pegg A. E. Polyamines as targets for therapeutic intervention. Annu Rev Pharmacol Toxicol. 1995;35:55–91. doi: 10.1146/annurev.pa.35.040195.000415. [DOI] [PubMed] [Google Scholar]
- Milam K. M., Hunter K. J., Deen D. F., Marton L. J. Reduction in cis-diamminedichloroplatinum(II)-induced cytotoxicity, sister chromatid exchange, and DNA interstrand cross-links in 9L cells treated with the polyamine biosynthesis inhibitor (2R,5R)-6-heptyne-2,5-diamine. Cancer Res. 1989 Dec 15;49(24 Pt 1):6945–6948. [PubMed] [Google Scholar]
- Oredsson S. M., Deen D. F., Marton L. J. Decreased cytotoxicity of cis-diamminedichloroplatinum(II) by alpha-difluoromethylornithine depletion of polyamines in 9L rat brain tumor cells in vitro. Cancer Res. 1982 Apr;42(4):1296–1299. [PubMed] [Google Scholar]
- Pegg A. E. Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy. Cancer Res. 1988 Feb 15;48(4):759–774. [PubMed] [Google Scholar]
- Pohjanpelto P., Nordling S., Knuutila S. Flow cytometric analysis of the cell cycle in polyamine-depleted cells. Cytometry. 1994 Aug 1;16(4):331–338. doi: 10.1002/cyto.990160407. [DOI] [PubMed] [Google Scholar]
- Pommier Y., Kerrigan D., Kohn K. Topological complexes between DNA and topoisomerase II and effects of polyamines. Biochemistry. 1989 Feb 7;28(3):995–1002. doi: 10.1021/bi00429a012. [DOI] [PubMed] [Google Scholar]
- Regenass U., Mett H., Stanek J., Mueller M., Kramer D., Porter C. W. CGP 48664, a new S-adenosylmethionine decarboxylase inhibitor with broad spectrum antiproliferative and antitumor activity. Cancer Res. 1994 Jun 15;54(12):3210–3217. [PubMed] [Google Scholar]
- Seidenfeld J., Barnes D., Block A. L., Erickson L. C. Comparison of DNA interstrand cross-linking and strand breakage by 1,3-bis(2-chloroethyl)-1-nitrosourea in polyamine-depleted and control human adenocarcinoma cells. Cancer Res. 1987 Sep 1;47(17):4538–4543. [PubMed] [Google Scholar]
- Seidenfeld J., Block A. L., Komar K. A., Naujokas M. F. Altered cell cycle phase distributions in cultured human carcinoma cells partially depleted of polyamines by treatment with difluoromethylornithine. Cancer Res. 1986 Jan;46(1):47–53. [PubMed] [Google Scholar]
- Seidenfeld J., Komar K. A., Naujokas M. F., Block A. L. Reduced cytocidal efficacy for adriamycin in cultured human carcinoma cells depleted of polyamines by difluoromethylornithine treatment. Cancer Res. 1986 Mar;46(3):1155–1159. [PubMed] [Google Scholar]
- Seidenfeld J., Sprague W. S. Comparisons between sensitive and resistant human tumor cell lines regarding effects of polyamine depletion on chloroethylnitrosourea efficacy. Cancer Res. 1990 Feb 1;50(3):521–526. [PubMed] [Google Scholar]
- Sherman S. E., Gibson D., Wang A. H., Lippard S. J. X-ray structure of the major adduct of the anticancer drug cisplatin with DNA: cis-[Pt(NH3)2(d(pGpG))]. Science. 1985 Oct 25;230(4724):412–417. doi: 10.1126/science.4048939. [DOI] [PubMed] [Google Scholar]
- Stjernborg L., Heby O., Mamont P., Persson L. Polyamine-mediated regulation of S-adenosylmethionine decarboxylase expression in mammalian cells. Studies using 5'-([(Z)-4-amino-2-butenyl]methylamino)-5'-deoxyadenosine, a suicide inhibitor of the enzyme. Eur J Biochem. 1993 Jun 15;214(3):671–676. doi: 10.1111/j.1432-1033.1993.tb17967.x. [DOI] [PubMed] [Google Scholar]
- Thomas T. J., Messner R. P. Structural specificity of polyamines in left-handed Z-DNA formation. Immunological and spectroscopic studies. J Mol Biol. 1988 May 20;201(2):463–467. doi: 10.1016/0022-2836(88)90155-6. [DOI] [PubMed] [Google Scholar]
- Xiao L., Swank R. A., Matthews H. R. Photoaffinity polyamines: sequence-specific interactions with DNA. Nucleic Acids Res. 1991 Jul 11;19(13):3701–3708. doi: 10.1093/nar/19.13.3701. [DOI] [PMC free article] [PubMed] [Google Scholar]



