Abstract
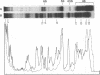
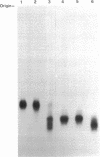
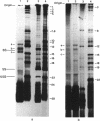
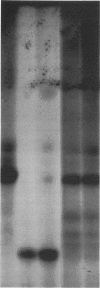
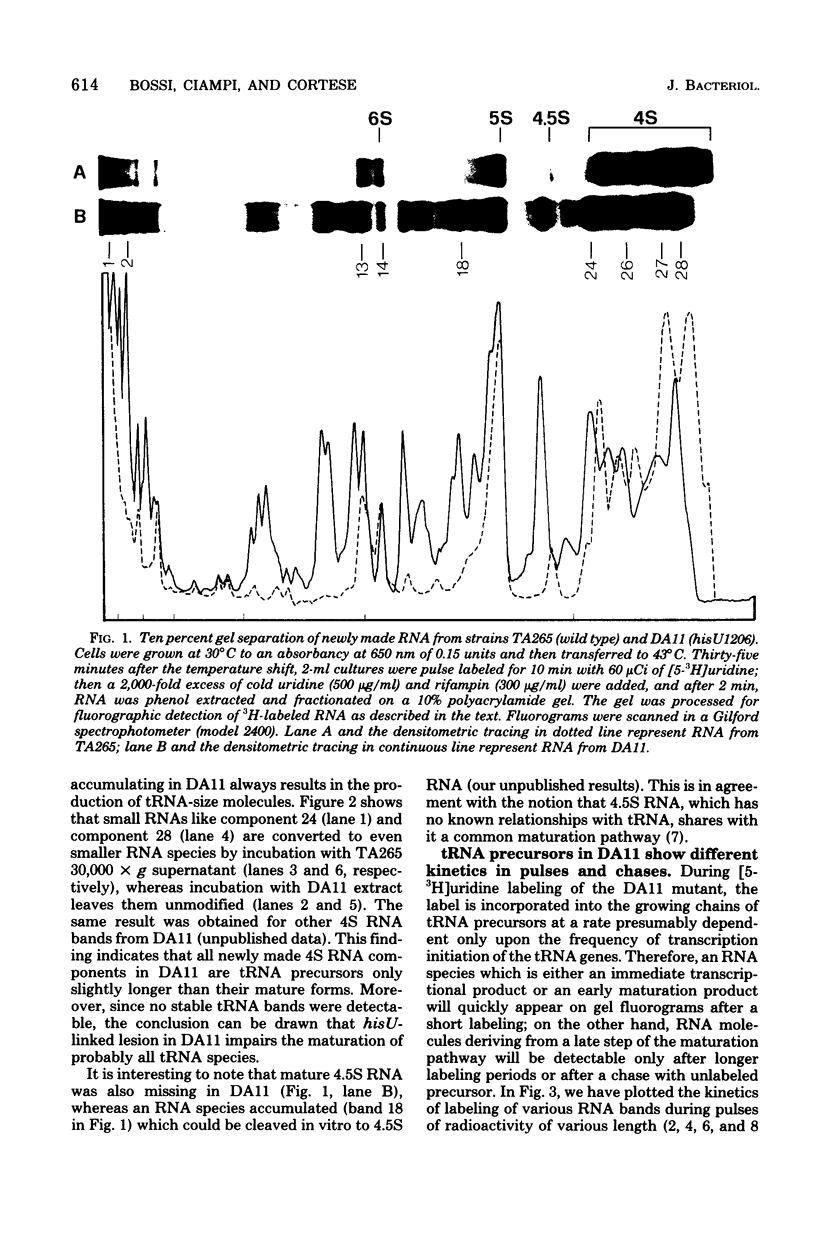
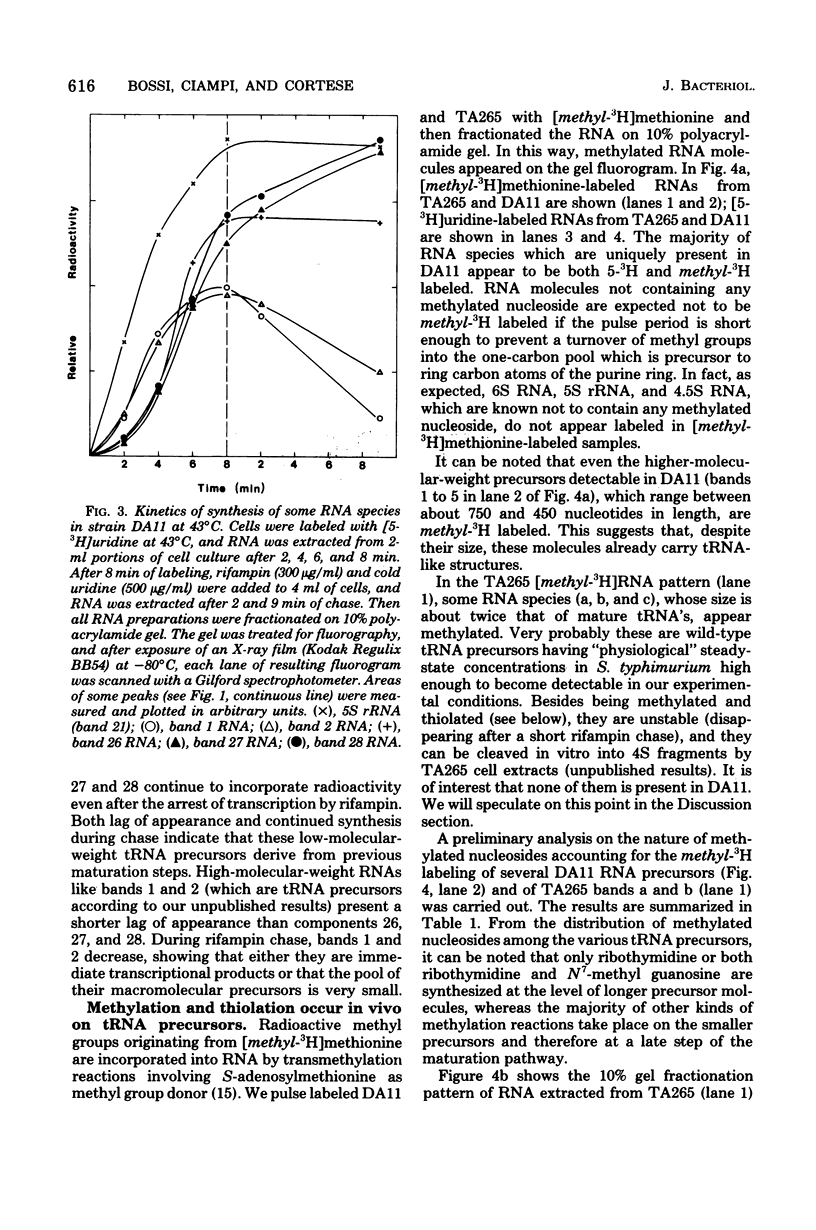
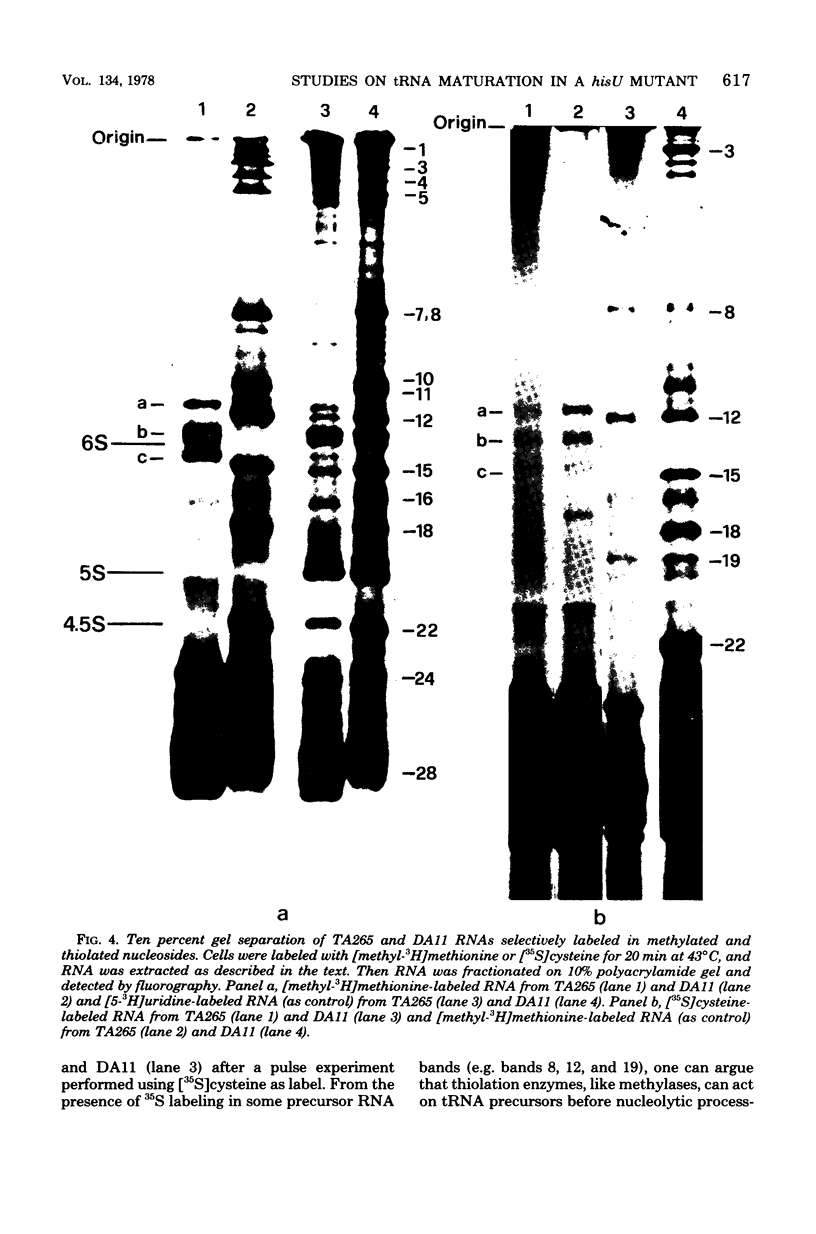
The DA11 mutant of Salmonella typhimurium, originally isolated as derepressed for the histidine operon, carries a temperature-dependent alteration in a nucleolytic enzyme specifically involved in the maturation of tRNA. As a consequence of this alteration, no detectable synthesis of any mature tRNA species occurs in DA11 upon shift at 43 degrees C, whereas many tRNA precursors, whose sizes range between 80 and 750 nucleotides, do accumulate. Kinetic studies on the synthesis and processing of these maturation intermediates show that these molecules represent different stages in the maturation pathway, most of them being the products of previous nucleolytic events. These RNA molecules are in vivo substrates of methylation and thiolation enzymes and can be cleaved in vitro to 4S RNA by wild-type but not by DA11 cell-free extract. Evidence is presented that DA11 is very probably a ribonuclease P mutant.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelson J., Fukada K., Johnson P., Lamfrom H., Nierlich D. P., Otsuka A., Paddock G. V., Pinkerton T. C., Sarabhai A., Stahl S. Bacteriophage T4 tRNAs: structure, genetics, and biosynthesis. Brookhaven Symp Biol. 1975 Jul;(26):77–88. [PubMed] [Google Scholar]
- Altman S., Bothwell A. L., Stark B. C. Processing of E. coli tRNA Tyr precursor RNA in vitro. Brookhaven Symp Biol. 1975 Jul;(26):12–25. [PubMed] [Google Scholar]
- Antón D. N. Histidine regulatory mutants in Salmonella typhimurium. V. Two new classes histidine regulatory mutants. J Mol Biol. 1968 May 14;33(3):533–546. doi: 10.1016/0022-2836(68)90304-5. [DOI] [PubMed] [Google Scholar]
- Blank H. U., Söll D. The nucleotide sequence of two leucine tRNA species from Escherichia coli K12. Biochem Biophys Res Commun. 1971 Jun 4;43(5):1192–1197. doi: 10.1016/0006-291x(71)90589-4. [DOI] [PubMed] [Google Scholar]
- Bossi L., Cortese R. Biosynthesis of tRNA in histidine regulatory mutants of Salmonella typhimurium. Nucleic Acids Res. 1977 Jun;4(6):1945–1956. doi: 10.1093/nar/4.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell A. L., Garber R. L., Altman S. Nucleotide sequence and in vitro processing of a precursor molecule to Escherichia coli 4.5 S RNA. J Biol Chem. 1976 Dec 10;251(23):7709–7716. [PubMed] [Google Scholar]
- Brenner M., Ames B. N. Histidine regulation in Salmonella typhimurium. IX. Histidine transfer ribonucleic acid of the regulatory mutants. J Biol Chem. 1972 Feb 25;247(4):1080–1088. [PubMed] [Google Scholar]
- Carbon J., Chang S., Kirk L. L. Clustered tRNA genes in Escherichia coli: transcription and processing. Brookhaven Symp Biol. 1975 Jul;(26):26–36. [PubMed] [Google Scholar]
- Edlin G., Maaloe O. Synthesis and breakdown of messenger RNA without protein synthesis. J Mol Biol. 1966 Feb;15(2):428–434. doi: 10.1016/s0022-2836(66)80118-3. [DOI] [PubMed] [Google Scholar]
- Guthrie C., Seidman J. G., Altman S., Barrell B. G., Smith J. D., McClain W. H. Identification of tRNA precursor molecules made by phage T4. Nat New Biol. 1973 Nov 7;246(149):6–11. doi: 10.1038/newbio246006a0. [DOI] [PubMed] [Google Scholar]
- Guthrie C., Seidman J. G., Comer M. M., Bock R. M., Schmidt F. J., Barrell B. G., McClain W. H. The biology of bacteriophage T4 transfer RNAs. Brookhaven Symp Biol. 1975 Jul;(26):106–123. [PubMed] [Google Scholar]
- Ikemura T., Dahlberg J. E. Small ribonucleic acids of Escherichia coli. I. Characterization by polyacrylamide gel electrophoresis and fingerprint analysis. J Biol Chem. 1973 Jul 25;248(14):5024–5032. [PubMed] [Google Scholar]
- Ikemura T., Shimura Y., Sakano H., Ozeki H. Precursor molecules of Escherichia coli transfer RNAs accumulated in a temperature-sensitive mutant. J Mol Biol. 1975 Jul 25;96(1):69–86. doi: 10.1016/0022-2836(75)90182-5. [DOI] [PubMed] [Google Scholar]
- Lewis J. A., Ames B. N. Histidine regulation in Salmonella typhimurium. XI. The percentage of transfer RNA His charged in vivo and its relation to the repression of the histidine operon. J Mol Biol. 1972 Apr 28;66(1):131–142. doi: 10.1016/s0022-2836(72)80011-1. [DOI] [PubMed] [Google Scholar]
- McClain W. H., Guthrie C., Barrell B. G. Eight transfer RNAs induced by infection of Escherichia coli with bacteriophage T4. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3703–3707. doi: 10.1073/pnas.69.12.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock G., Abelson J. Sequence of T4, T2 and T6 bacteriophage species I RNA and specific cleavage by an E. coli endonuclease. Nat New Biol. 1973 Nov 7;246(149):2–6. doi: 10.1038/newbio246002a0. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Resolution of multiple ribonucleic acid species by polyacrylamide gel electrophoresis. Biochemistry. 1967 Jun;6(6):1818–1827. doi: 10.1021/bi00858a033. [DOI] [PubMed] [Google Scholar]
- Randerath K. An evaluation of film detection methods for weak beta-emitters, particularly tritium. Anal Biochem. 1970 Mar;34:188–205. doi: 10.1016/0003-2697(70)90100-4. [DOI] [PubMed] [Google Scholar]
- Rogg H., Brambilla R., Keith G., Staehelin M. An improved method for the separation and quantitation of the modified nucleosides of transfer RNA. Nucleic Acids Res. 1976 Jan;3(1):285–295. doi: 10.1093/nar/3.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakano H., Shimura Y. Sequential processing of precursor tRNA molecules in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3369–3373. doi: 10.1073/pnas.72.9.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakano H., Yamada S., Ikemura T., Shimura Y., Ozeki H. Temperature sensitive mutants of Escherichia coli for tRNA synthesis. Nucleic Acids Res. 1974 Mar;1(3):355–371. doi: 10.1093/nar/1.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedl P., Primakoff P. Mutants of Escherichia coli thermosensitive for the synthesis of transfer RNA. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2091–2095. doi: 10.1073/pnas.70.7.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedl P., Primakoff P., Roberts J. Processing of E. coli tRNA precursors. Brookhaven Symp Biol. 1975 Jul;(26):53–76. [PubMed] [Google Scholar]
- Seidman J. G., Barrell B. G., McClain W. H. Five steps in the conversion of a large precursor RNA into bacteriophage proline and serine transfer RNAs. J Mol Biol. 1975 Dec 25;99(4):733–760. doi: 10.1016/s0022-2836(75)80182-3. [DOI] [PubMed] [Google Scholar]
- Singer C. E., Smith G. R., Cortese R., Ames B. N. [Mutant tRNA His ineffective in repression and lacking two pseudouridine modifications]. Nat New Biol. 1972 Jul 19;238(81):72–74. doi: 10.1038/newbio238072a0. [DOI] [PubMed] [Google Scholar]
- Smith J. D. Mutants which allows accumulation of tRNATyr precursor molecules. Brookhaven Symp Biol. 1975 Jul;(26):1–11. [PubMed] [Google Scholar]
- Smith J. D. Transcription and processing of transfer RNA precursors. Prog Nucleic Acid Res Mol Biol. 1976;16:25–73. doi: 10.1016/s0079-6603(08)60755-2. [DOI] [PubMed] [Google Scholar]
- Squires C., Carbon J. Normal and mutant glycine transfer RNAs. Nat New Biol. 1971 Oct 27;233(43):274–277. doi: 10.1038/newbio233274a0. [DOI] [PubMed] [Google Scholar]
- Wilson J. H., Kim J. S., Abelson J. N. Bacteriophage T4 transfer RNA. 3. Clustering of the genes for the T4 transfer RNA's. J Mol Biol. 1972 Nov 28;71(3):547–556. doi: 10.1016/s0022-2836(72)80022-6. [DOI] [PubMed] [Google Scholar]