Abstract
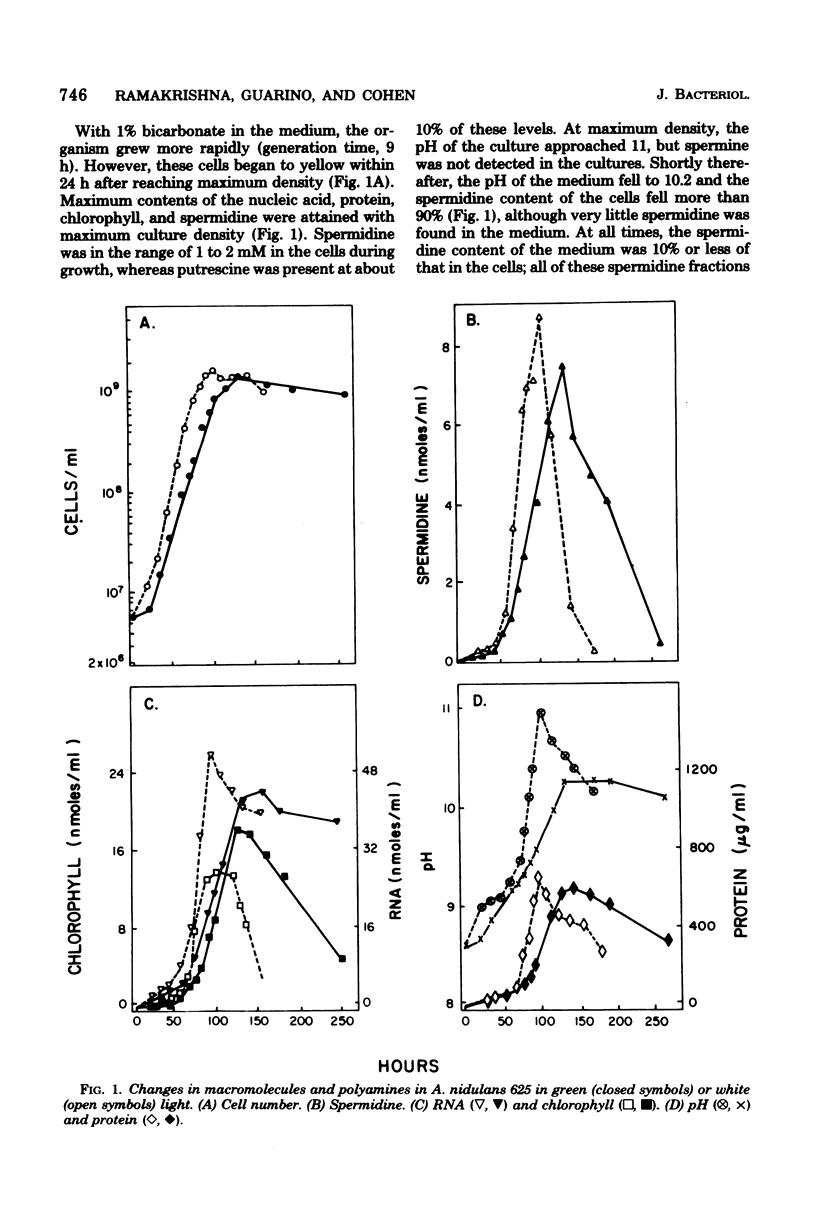
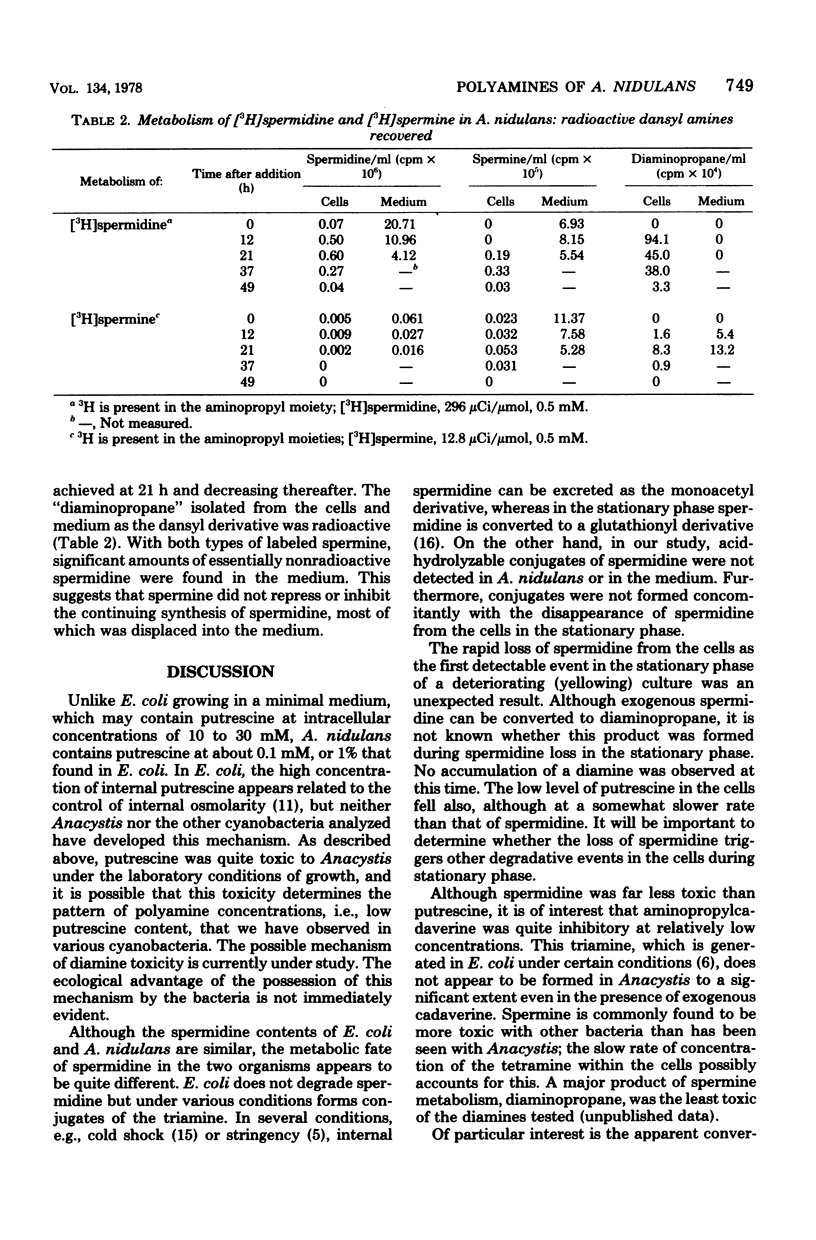
Several biochemical parameters, including that of polyamine content, accompanying the growth of the cyanobacterium Anacystis nidulans were studied. At all stages of growth under autotrophic conditions, the organisms were found to be rich in spermidine and lacking in spermine, as is typical of procaryotic organisms. The cells were quite low in putrescine, and no unusual polyamine was observed to be present as a major component. Conjugated polyamines were not detected in the cultures. At maximal culture density, the levels of spermidine, DNA, RNA, protein, and chlorophyll were also maximal. Shortly after the inception of the stationary phase, the spermidine content of the cells was the first parameter observed to decrease in cultures which were shortly to become yellow. Spermidine lost from the cells was not recovered in the medium in a free or conjugated form. This indication of degradation of spermidine was studied by the addition of polyamines to growing cultures. Exogenous spermidine and spermine were found to be metabolized rapidly by the organisms, of which diaminopropane was one product. Putrescine was found to be markedly toxic, whereas spermidine, some other triamines, and spermine were much less toxic.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacchi C. J., Lipschik G. Y., Nathan H. C. Polyamines in trypanosomatids. J Bacteriol. 1977 Aug;131(2):657–661. doi: 10.1128/jb.131.2.657-661.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman W. H., Tabor C. W., Tabor H. Spermidine biosynthesis. Purification and properties of propylamine transferase from Escherichia coli. J Biol Chem. 1973 Apr 10;248(7):2480–2486. [PubMed] [Google Scholar]
- Cohen S. S., Hoffner N., Jansen M., Moore M., Raina A. POLYAMINES, RNA SYNTHESIS, AND STREPTOMYCIN LETHALITY IN A RELAXED MUTANT OF E. coli STRAIN 15 TAU. Proc Natl Acad Sci U S A. 1967 Mar;57(3):721–728. doi: 10.1073/pnas.57.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBIN D. T., ROSENTHAL S. M. The acetylation of polyamines in Escherichia coli. J Biol Chem. 1960 Mar;235:776–782. [PubMed] [Google Scholar]
- Dion A. S., Cohen S. S. Polyamine stimulation of nucleic acid synthesis in an uninfected and phage-infected polyamine auxotroph of Escherichia coli K12 (arginine-agmatine ureohydrolase-putrescine-spermidine-lysine-cadaverine). Proc Natl Acad Sci U S A. 1972 Jan;69(1):213–217. doi: 10.1073/pnas.69.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihlenfeldt M. J., Gibson J. CO2 fixation and its regulation in Anacystis nidulans (Synechococcus). Arch Microbiol. 1975;102(1):13–21. doi: 10.1007/BF00428339. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- Munro G. F., Hercules K., Morgan J., Sauerbier W. Dependence of the putrescine content of Escherichia coli on the osmotic strength of the medium. J Biol Chem. 1972 Feb 25;247(4):1272–1280. [PubMed] [Google Scholar]
- Nickerson K. W., Dunkle L. D., Van Etten J. L. Absence of spermine in filamentous fungi. J Bacteriol. 1977 Jan;129(1):173–176. doi: 10.1128/jb.129.1.173-176.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Kunisawa R., Mandel M., Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev. 1971 Jun;35(2):171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W. The effects of temperature on the acetylation of spermidine. Biochem Biophys Res Commun. 1968 Feb 26;30(4):339–342. doi: 10.1016/0006-291x(68)90747-x. [DOI] [PubMed] [Google Scholar]
- Tabor H., Tabor C. W. Glutathionylspermidine in Escherichia coli. Ital J Biochem. 1976 Jan-Feb;25(1):70–76. [PubMed] [Google Scholar]


