Abstract
Pyrobaculum islandicum uses iron, thiosulfate, and elemental sulfur for anaerobic respiration, while Pyrobaculum aerophilum uses iron and nitrate; however, the constraints on these processes and their physiological mechanisms for iron and sulfur reduction are not well understood. Growth rates on sulfur compounds are highest at pH 5 to 6 and highly reduced (<−420-mV) conditions, while growth rates on nitrate and iron are highest at pH 7 to 9 and more-oxidized (>−210-mV) conditions. Growth on iron expands the known pH range of growth for both organisms. P. islandicum differs from P. aerophilum in that it requires direct contact with insoluble iron oxide for growth, it did not produce any extracellular compounds when grown on insoluble iron, and it lacked 2,6-anthrahydroquinone disulfonate oxidase activity. Furthermore, iron reduction in P. islandicum appears to be completely independent of c-type cytochromes. Like that in P. aerophilum, NADH-dependent ferric reductase activity in P. islandicum increased significantly in iron-grown cultures relative to that in non-iron-grown cultures. Proteomic analyses showed that there were significant increases in the amounts of a putative membrane-bound thiosulfate reductase in P. islandicum cultures grown on thiosulfate relative to those in cultures grown on iron and elemental sulfur. This is the first evidence of this enzyme being used in either a hyperthermophile or an archaeon. Pyrobaculum arsenaticum and Pyrobaculum calidifontis also grew on Fe(III) citrate and insoluble iron oxide, but only P. arsenaticum could grow on insoluble iron without direct contact.
It is estimated that as much as one-fifth of the earth's biomass consists of microorganisms living deep within the crust in the so-called subsurface biosphere (33). Organisms found in these deep habitats include thermophiles (4, 14, 18) and hyperthermophiles (16, 28). They use nitrogen, sulfur, and iron compounds as well as O2 as their terminal electron acceptor, but the constraints on these processes and the mechanisms of iron and sulfur reduction, especially at high temperatures, are not well understood. Hyperthermophilic archaea in the genus Pyrobaculum have been found in fluids from deep geothermal pools (e.g., Pyrobaculum islandicum) (10, 28) and in hot springs (e.g., Pyrobaculum aerophilum) (2, 11, 24, 32). P. islandicum uses Fe(III), S2O32−, and S0 as terminal electron acceptors (10, 13), while P. aerophilum uses Fe(III), NO3−, and low O2 (13, 32). The ubiquity of Pyrobaculum species in terrestrial hydrothermal environments, their metabolic diversity, and the availability of four complete Pyrobaculum genome sequences make these organisms both environmentally relevant and ideal for physiology and ecology studies.
Unlike most other hyperthermophilic iron reducers, P. islandicum and P. aerophilum use both soluble [Fe(III) citrate] and insoluble [Fe(III) oxide hydroxide] forms of iron as electron acceptors (13, 31). NADH-dependent ferric reductase activity is found primarily in the soluble protein fractions of P. islandicum and P. aerophilum, and both organisms show evidence for b- and c-type cytochromes in their membranes (6, 8). P. aerophilum does not have polyheme c-type cytochromes, which sets it apart from iron reduction in Shewanella and Geobacter, nor does it require direct contact with insoluble Fe(III) oxide hydroxide for growth (8).
This study sought to determine how environmental factors such as pH and reduction potential influence anaerobic respiration in P. islandicum and P. aerophilum. It also investigates whether P. islandicum, Pyrobaculum arsenaticum, and Pyrobaculum calidifontis require direct contact with insoluble iron and sulfur for respiration. Furthermore, cell-free spent media from P. islandicum and P. aerophilum grown with different terminal electron acceptors were compared using liquid chromatography (LC) and analyzed using tandem mass spectrometry (MS-MS) to identify compounds that accumulate in the supernatants during growth that could potentially serve as extracellular mediators for the reduction of insoluble iron. Finally, it analyzes membrane protein and c-type cytochrome compositions in P. islandicum following growth on iron, thiosulfate, and sulfur for respiratory proteins specific to these metabolisms.
MATERIALS AND METHODS
Organisms used.
P. islandicum DSM 4184, P. aerophilum DSM 7523, P. arsenaticum DSM 13514, and P. calidifontis JCM11548 were used for this study. P. islandicum was kindly provided by Kazem Kashefi and Derek Lovley (Department of Microbiology, University of Massachusetts, Amherst, MA). P. aerophilum was purchased from the Deutsche Sammlung von Mikroorganismen und Zellkulturen (Braunschweig, Germany). P. arsenaticum and P. calidifontis were kindly provided by Todd Lowe (Department of Biomolecular Engineering, University of California, Santa Cruz, CA).
Growth conditions.
The media for P. islandicum, P. arsenaticum, and P. calidifontis growth were based on those described previously (25). The media for P. aerophilum growth were modified from Kashefi medium C, which was described previously (8, 12) (K. Kashefi, personal communication). P. islandicum, P. arsenaticum, and P. calidifontis were grown on 0.05% (wt/vol) casein hydrolysate (enzymatic; Difco) and 0.02% (wt/vol) yeast extract (Difco). P. aerophilum was grown on 0.1% each of casein hydrolysate and yeast extract. The terminal electron acceptors were 100 mM Fe(III) oxide hydroxide, 20 mM Fe(III) citrate, 0.1% (wt/vol) elemental sulfur (S0), 8 mM Na2S2O3, and 10 mM KNO3. The pHs of the media were adjusted to 6.00 ± 0.05 and 6.80 ± 0.05 at room temperature for P. islandicum and P. aerophilum, respectively, unless otherwise stated. Cysteine-HCl (0.5 mM) was added as the reducing agent to remove residual O2. Fermentor-grown cultures were grown in a 20-liter fermentor that was flushed with argon at a flow rate of 30 ml min−1, stirred at 120 to 150 rpm, and heated to 95°C ± 0.1°C. For P. aerophilum, the pHs of the Fe(III) citrate and nitrate media at 95°C were maintained at 6.0 ± 0.1 and 6.3 ± 0.1, respectively, using an automated pH control system. For P. islandicum, the pHs of the Fe(III) citrate, thiosulfate, and sulfur media at 95°C were maintained at 5.7 ± 0.1. At various times during growth, an aliquot was removed to measure the concentrations of cells by using a Petroff-Hausser counting chamber and phase-contrast light microscopy. The specific growth rates (k) of the cultures were determined by a best-fit curve through the logarithmic portion of the growth data. Cells from the fermentor were harvested when they reached late logarithmic growth phase (∼108 cells ml−1) as described previously (8).
Media (50 ml) for the pH and reduction potential experiments were contained in 120-ml serum bottles sealed with a butyl rubber stopper and flushed with Ar. For the pH experiment, media were pH balanced at pH 5 and 6 using 5 mM MES (morpholineethanesulfonic acid) buffer, at 7 and 8 using 20 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)] buffer, and at 8.5 and 9.0 using 30 mM NaHCO3 buffer, all using 0.5 mM cysteine-HCl as the reducing agent. For the reduction potential experiments, the media were reduced with 2 mM Na2S·9H2O (−571 mV), H2, six to eight palladium pellets (−420 mV), 3.2 mM dithiothreitol (−330 mV), and 0.5 mM cysteine-HCl (−210 mV), with reduction potentials as indicated previously (7). The media were also degassed and flushed with argon without the addition of a reducing agent. The resulting reduction potentials of each unreduced medium were determined using an oxidation reduction potential probe at room temperature. Cultures were incubated at 95°C in duplicate, and their growth rates were determined as described above.
Iron barrier and spent supernatant analyses.
P. islandicum, P. arsenaticum, and P. calidifontis were grown in media with Fe(III) oxide hydroxide or S0 (P. islandicum only) that were contained within dialysis tubing barriers (12,000- to 14,000-Da pore size; SpectraPor) as described previously (8) in order to determine whether direct contact with either insoluble electron acceptor was necessary for growth. It was previously shown that the dialysis tubing maintains its integrity when incubated at 95°C (8). All samples were incubated at 95°C with and without the dialysis tubing, and growth rates were determined as described above.
For supernatant analyses, P. aerophilum was grown in media containing Fe(III) oxide hydroxide, Fe(III) citrate, and nitrate; P. islandicum was grown in media containing Fe(III) oxide hydroxide, Fe(III) citrate, thiosulfate, and S0; and uninoculated media were incubated for 24 h at 95°C. Cells were removed from the spent media by centrifugation and filtration as described previously (8). Supernatant profiles were determined using a Hewlett-Packard 1100 series high-performance LC separation system equipped with a variable-wavelength UV-visible light absorption detector. Cell-free spent supernatant (20 μl) was loaded onto a 150-mm by 3.9-mm Nova-Pak C18 column (Waters) at room temperature at a flow rate of 1 ml min−1 and eluted using a gradient of two elution solutions. Eluent A consisted of 0.1% trifluoroacetic acid in water, and eluent B consisted of 0.1% trifluoroacetic acid in acetonitrile. The gradient was performed by increasing eluent B from 1% to 100% over 40 min. Additional chemical information about the separated compounds was gathered using LC coupled to MS (LC-MS) and LC-MS-MS. The samples were reinjected onto and eluted from the same C18 column by using the same mobile phase gradient described previously, but electrospray ionization MS on a Bruker Esquire-LC quadrupole ion trap MS was used for detection.
Protein analyses and enzyme activity assays.
Cell material from the fermentor was separated into cytoplasmic and membrane fractions as described previously (1, 8). The protein concentrations of the whole-cell extract and the cytoplasmic fractions were determined spectrophotometrically using a protein determination kit (Bio-Rad) based on the Bradford assay (5). The protein concentrations of the membrane fractions were determined using a kit for detergent-containing samples (DC protein assay kit; Bio-Rad). The latter kit could not be used to determine the protein concentrations of the whole-cell extract and cytoplasmic fractions from iron-grown cells, due to high background reactivity. Bovine serum albumin was used as the protein standard for both kits. Protein fractions that were not used immediately for analysis were frozen in liquid N2 and stored at −80°C.
Equal amounts of protein from the membrane fractions were separated by electrophoresis and visualized separately using silver and Coomassie blue staining. A 120-kDa protein band stained with Coomassie from thiosulfate-grown cells was excised from the gel and digested overnight with trypsin (Promega) as described previously (26). The digested peptide fragments were analyzed using a matrix-assisted laser desorption ionization-time of flight MS. The masses of the fragments were then correlated with the P. islandicum genome for protein identification using the Web-based program Aldente (http://ca.expasy.org/tools/aldente/). Equal amounts of protein from the membrane fractions were separated by electrophoresis without a reducing agent, and proteins containing the c-type heme were identified as described previously (29). Ferric reductase, 2,6-anthrahydroquinone disulfonate (AHDS) oxidase, and malate dehydrogenase (MDH) activities were determined spectrophotometrically as described previously (8, 27).
RESULTS
Growth rates at various pHs and reduction potentials.
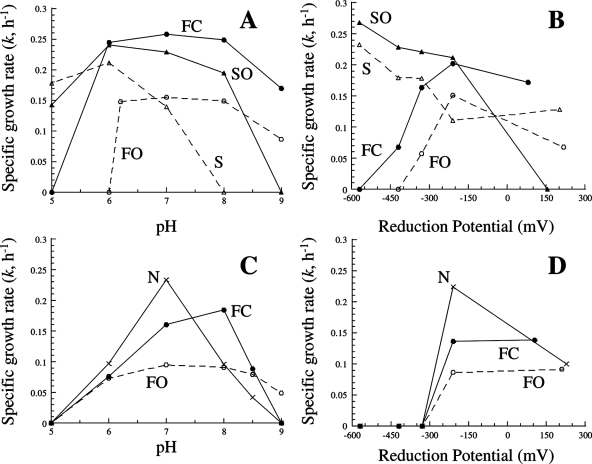
The growth rates of P. islandicum and P. aerophilum at various pHs and reduction potentials differed significantly with the terminal electron acceptor used (Fig. 1). For P. islandicum, growth on S0 and thiosulfate occurred at pH 5, 6, and 7 and at pH 6, 7, and 8, respectively (Fig. 1A). Growth on Fe(III) citrate and Fe(III) oxide hydroxide occurred at pH 6, 7, 8, and 9 and at pH 6.2, 7, 8, and 9, respectively [the Fe(III) oxide hydroxide turned to clay below pH 6.2]. For P. aerophilum, growth on nitrate occurred at pH 6, 7, 8, and 8.5, with a clear optimum at pH 7 (Fig. 1C). Similar to what was found for P. islandicum, growth on Fe(III) citrate and Fe(III) oxide hydroxide occurred at pH 6, 7, 8, and 8.5 and at pH 6, 7, 8, 8.5, and 9, respectively. In general, growth on sulfur was more favorable at slightly acidic pHs, growth on nitrate at neutral pHs, and growth on iron at neutral to slightly basic pHs. The final pHs following growth did not differ significantly from their initial values.
FIG. 1.
k values of P. islandicum (A, B) and P. aerophilum (C, D) grown on 20 mM Fe(III) citrate (•; FC), 100 mM Fe(III) oxide hydroxide (○; FO), 8 mM Na2S2O3 (▴; SO), 0.1% elemental sulfur (▵; S), and 10 mM KNO3 (×; N) at different pHs (A, C) and reduction potentials (B, D). Soluble terminal electron acceptors are shown with solid lines and insoluble acceptors with dashed lines.
Reduction potential had a more pronounced effect on growth rates. For P. islandicum, growth rates on S0 and thiosulfate were highest at the lowest reduction potentials and decreased with increasing potential. Fe(III) citrate and Fe(III) oxide hydroxide both react abiotically with sulfide to form a black precipitate at −571 mV. Growth on Fe(III) citrate and Fe(III) oxide hydroxide occurred between −420 and 79 mV and between −330 and 217 mV, respectively (Fig. 1B). Growth was highest when a mild reducing agent (cysteine) was used. For P. aerophilum, there was no growth below −210 mV (Fig. 1D). Growth on nitrate was highest at −210 mV and decreased significantly when no reducing agent was added (228 mV). The absence of a reducing agent did not significantly affect iron reduction relative to growth with cysteine. Sulfur reduction was clearly favored under more-highly reducing conditions, while nitrate and iron reduction were more favored under more-oxidizing but anaerobic conditions.
Iron and sulfur barrier experiments.
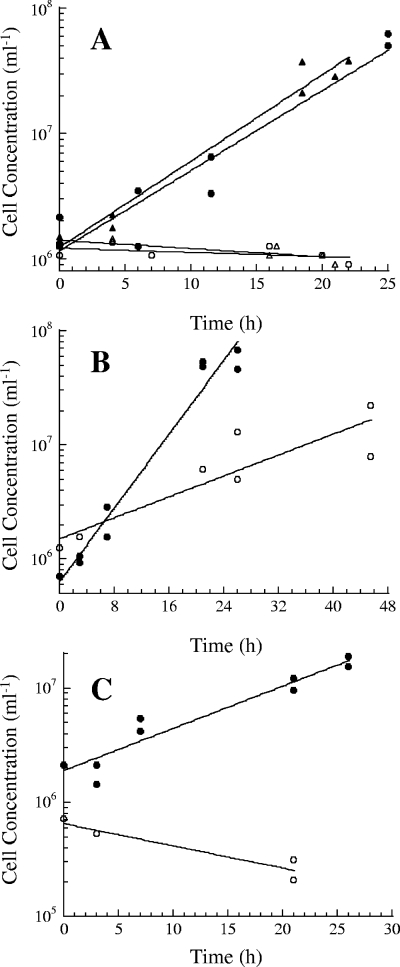
The doubling times of P. islandicum grown on Fe(III) oxide hydroxide and S0 without a barrier were 4.7 h (k = 0.15 h−1 ± 0.05 h−1) and 4.4 h (k = 0.16 h−1 ± 0.02 h−1), respectively (Fig. 2A). However, P. islandicum was unable to grow on either insoluble iron or insoluble sulfur when separated from it by the barrier, indicating that P. islandicum requires direct contact with both electron acceptors for growth. P. arsenaticum can grow on insoluble iron when a barrier is present, albeit at a lower growth rate (Fig. 2B), but P. calidifontis cannot (Fig. 2C). The doubling times of P. arsenaticum grown without and with the barrier were 3.7 h (k = 0.19 h−1 ± 0.03 h−1) and 13.0 h (k = 0.05 h−1 ± 0.02 h−1), respectively. The doubling time of P. calidifontis grown without the barrier was 8.1 h (k = 0.09 h−1 ± 0.02 h−1). Therefore, the ability of Pyrobaculum to grow on insoluble iron without direct contact varies with strains.
FIG. 2.
(A) Growth of P. islandicum on 100 mM Fe(III) oxide hydroxide without (•) and with (○) the barrier and on 0.1% elemental sulfur without (▴) and with (▵) the barrier. (B, C) Growth of P. arsenaticum (B) and P. calidifontis (C) on 100 mM Fe(III) oxide hydroxide without (•) and with (○) the barrier.
Cell-free supernatant analyses.
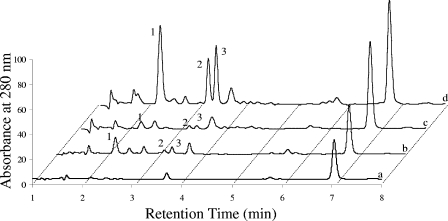
The chromatograms of P. islandicum cell-free spent supernatants from cultures grown on insoluble iron, Fe(III) citrate, thiosulfate, and S0 did not show any significant differences between them or with their respective uninoculated controls. The chromatograms for Fe(III) oxide hydroxide spent media from P. islandicum and P. aerophilum between 1 and 8 min, which is where the largest insoluble iron-specific peaks were found previously in P. aerophilum (8), were compared (Fig. 3). The 2.2-, 3.2-, and 3.4-min peaks (labeled 1 to 3) found in P. aerophilum were largely absent from P. islandicum. Peaks with the same retention times were also found in P. aerophilum uninoculated medium, but the UV absorbance peak areas (means ± standard deviations) were 10.2- ± 0.5-, 12.6- ± 0.3-, and 16.3- ± 0.6-fold higher in the experimental samples for the 2.2-, 3.2-, and 3.4-min peaks, respectively. The 3.4-min peak was also observed in the supernatants of the cultures grown on Fe(III) citrate and nitrate (8), but its concentrations, based on peak area, were about 50% and 10%, respectively, of its concentration in the Fe(III) oxide hydroxide samples. This is despite the fact that the maximum cell concentration for cultures grown on insoluble iron is only half that of cultures grown on soluble iron and nitrate.
FIG. 3.
Absorbances at 280 nm following separation by LC of soluble compounds in cell-free spent supernatants from P. islandicum (b) and P. aerophilum (d) cultures grown on 100 mM Fe(III) oxide hydroxide and supernatants from uninoculated P. islandicum (a) and P. aerophilum (c) media. The numbers (1 to 3) alongside the peaks at 2.2, 3.2, and 3.4 min indicate the peaks of interest.
Additional chemical information for the 3.2- and 3.4-min peaks was gathered by LC-MS-MS. MS-MS spectra show that uridine and cytidine coelute in the 3.2-min peak while MS-MS and triple-MS (adenosine only) spectra show that adenosine and guanosine coelute in the 3.4-min peak (see Fig. S1 to S4 in the supplemental material). Extracted ion chromatography showed that the amounts of uridine, cytidine, adenosine, and guanine in the spent media were 3- ± 1-, 2.1- ± 0.5-, 6.5- ± 0.3-, and 5- ± 2-fold higher, respectively, than that in the uninoculated control. The compound in the 2.2-min peak was not ionized by electrospray ionization.
Membrane protein composition.
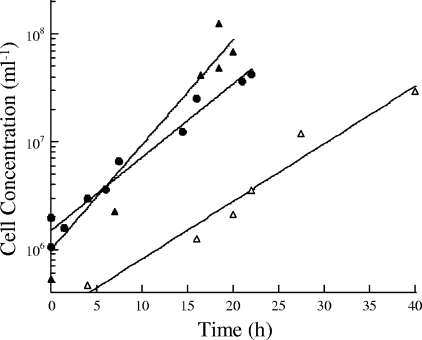
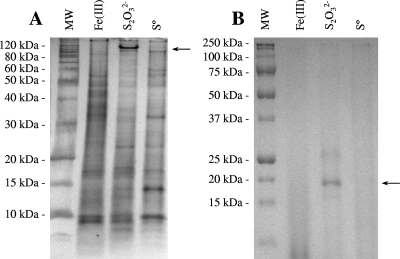
The doubling times of P. islandicum in the fermentor were 4.4 h (k = 0.16 h−1 ± 0.02 h−1), 3.1 h (k = 0.22 h−1 ± 0.05 h−1), and 5.6 h (k = 0.12 h−1 ± 0.04 h−1) for growth on Fe(III) citrate, thiosulfate, and S0, respectively (Fig. 4). The membrane protein composition had some significant changes with the terminal electron acceptor, as seen by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Fig. 5A). A band with a mass of 120 kDa that was absent from the other two fractions was observed in thiosulfate-grown cultures. Tryptic digestion and peptide mass fingerprinting of the 120-kDa band resulted in 17 well-defined peaks from the MS. Seven of the fragments matched (± 1 Da) those predicted for the catalytic subunit of thiosulfate reductase (Pisl_0266; GenBank accession no. CP000504), which has a predicted mass of 118 kDa (see Table S1 in the supplemental material). Eight fragments matched those predicted for a membrane-bound solute binding protein (Pisl_0913) with a predicted mass of 98 kDa but are believed to be a contaminant (see Table S1 in the supplemental material).
FIG. 4.
Growth of P. islandicum in the 20-liter fermentor on either 20 mM Fe(III) citrate (•), 8 mM Na2S2O3 (▴), or 0.1% elemental sulfur (▵).
FIG. 5.
Gel electrophoresis and silver staining (A) or heme staining (B) of P. islandicum membrane fractions prepared from Fe(III) citrate-, thiosulfate-, and elemental sulfur-grown cultures. (A) The protein band used for peptide mass fingerprinting is indicated by the arrow, and the molecular masses of the molecular size standard proteins (MW lane) are shown on the left. The gel contained 8% polyacrylamide. (B) The heme-stained protein band is identified by an arrow, and the molecular masses of prestained standard proteins (MW lane) are shown on the left.
The heme stain suggests that there is a c-type cytochrome protein (and possibly a second, weaker band) that is higher in relative abundance in membrane fractions from cultures grown only on thiosulfate (Fig. 5B). There was no heme-stained band in the membranes of iron-grown cells. The mass of the unreduced heme-stained protein seen in thiosulfate-grown cells was 19 kDa, which is lower than those of the two putative c-type cytochrome proteins found in P. aerophilum, suggesting that it is a different protein. The two putative c-type cytochromes in P. aerophilum (PAE1347 and PAE3598; GenBank accession no. AE009441) do not have any homologs within the P. islandicum genome. There are three open reading frames within the P. islandicum genome sequence (Pisl_1195, Pisl_1342, and Pisl_1348) that code for proteins containing one CXXCH motif, indicative of c-type cytochromes, and have a membrane protein signal peptide sequence, as determined by Web-based search programs (SignalP 3.0 [http://www.cbs.dtu.dk/services/SignalP/] and SOSUI [http://bp.nuap.nagoya-u.ac.jp/sosui/]). However, they all code for proteins whose masses are greater than 40 kDa. There are no open reading frames in the P. islandicum genome that have more than one CXXCH motif. A search of the P. arsenaticum and P. calidifontis genome sequences (GenBank accession no. CP000660 and CP000561, respectively) shows that both organisms also have putative membrane-bound c-type cytochromes with only a single CXXCH motif. Unlike the other three Pyrobaculum species, P. calidifontis does possess a putative membrane-bound polyheme c-type cytochrome (Pcal_1497) with eight predicted heme sites and a membrane signal peptide sequence. It shows highest homology with polyheme c-type cytochromes found in the mesophilic bacteria Shewanella (SO_1427; E value = 5 × 10−9) and Geobacter (GSU2645; E value = 7 × 10−8).
Enzyme activities.
The washing procedure was effective at removing cytoplasmic protein from the membrane fraction, as judged by the amounts of MDH activity in the various fractions (Table 1). P. islandicum MDH is a soluble protein (34) and was used as a marker to establish that the wash protocol provided complete separation of the cytoplasmic proteins from the membrane proteins. Relative to MDH activity in the whole-cell extract, most of the MDH activity was recovered in the cytoplasmic fraction, with most of the remainder lost in the pellet wash steps. Little to no MDH activity was measured in the membrane fraction.
TABLE 1.
Specific activities of NADH-dependent ferric reductase, AHDS oxidase, and malate dehydrogenase at 80°C in the cellular fractions from P. islandicum cells grown on Fe(III) citrate and thiosulfate
| Fraction | Sp act (U mg−1) (mean ± SD) for:
|
|||||
|---|---|---|---|---|---|---|
| Ferric reductase
|
AHDS oxidase
|
Malate dehydrogenase
|
||||
| Iron grown | Thiosulfate grown | Iron grown | Thiosulfate grown | Iron grown | Thiosulfate grown | |
| Whole-cell extract | 0.24 ± 0.07 | 0.03 ± 0.00 | 0.15 ± 0.02 | 0.03 ± 0.01 | 1.59 ± 1.25 | 1.32 ± 0.18 |
| Cytoplasm | 0.57 ± 0.03 | 0.07 ± 0.02 | 0.06 ± 0.06 | 0.07 ± 0.07 | 4.08 ± 0.27 | 3.33 |
| Membrane | 0.20 ± 0.04 | 0.03 ± 0.00 | 0.02 ± 0.01 | 0.04 ± 0.02 | 0.01 ± 0.00 | NDa |
ND, no activity detected.
The NADH-dependent ferric reductase activities in P. islandicum whole-cell extracts, the cytoplasmic fraction, and the membrane fraction were seven- to eightfold higher in iron-grown cells than in thiosulfate-grown cells, suggesting that the activity is regulated (Table 1). The highest specific activities were measured in the cytoplasmic fraction. The oxidation rates of AHDS relative to those of AQDS (anthraquinone-2,6-disulfonate) were low throughout the samples. The AHDS oxidase activities in the cytoplasmic and membrane fractions from iron- and thiosulfate-grown cells were not significantly different. Relative to AHDS oxidase activities measured in P. aerophilum (8), there does not appear to be any significant AHDS oxidase activity in P. islandicum.
DISCUSSION
Pyrobaculum species are capable of several forms respiration over wide ranges of pHs and reduction potentials, which suggests that these organisms respond to geochemical fluctuations within their native environments. The influence of pH and reduction potential on respiration is generally overlooked in most studies. All four Pyrobaculum species examined can reduce both Fe(III) citrate and Fe(III) oxide hydroxide. They are generally unique among hyperthermophilic Archaea in their abilities to reduce soluble iron and their production of a nonmagnetic end product following reduction of insoluble iron. However, there are significant differences between Pyrobaculum species in their abilities to reduce iron. Only two of the four species examined can reduce iron without direct contact. Only one species examined has a putative membrane-bound polyheme c-type cytochrome, while the others have only monoheme c-type cytochromes. Iron reduction in P. islandicum does not appear to involve any c-type cytochromes. Therefore, Pyrobaculum provides an additional model of microbial iron reduction that differs from those for Shewanella and Geobacter.
This study demonstrates the importance of pH and reduction potential for respiration. P. islandicum was characterized using S0 as the terminal electron acceptor and sulfide as the reducing agent (10). Under these conditions, it grew between pH 5 and 7, and our results agree with this finding. However, the ability of the organism to grow up to pH 9 under more-oxidized conditions when growing on iron broadens the potential habitats where this organism may be found and potentially demonstrates how environmental conditions may dictate which form of respiration occurs. The most pronounced environmental effect on anaerobic respiration was reduction potential. This suggests that in highly sulfidic environments where the reduction potential is very negative, iron and nitrate reduction by P. aerophilum, P. islandicum, and potentially other hyperthermophiles is inhibited while sulfur reduction is favored. In anoxic environments without sulfide or other strong reducing agents, the more positive reduction potential will favor nitrate and iron reduction.
One aspect of dissimilatory iron-reducing bacteria that is frequently studied is their abilities to reduce insoluble iron oxides without direct contact. Shewanella is generally capable of iron reduction without direct contact by producing an extracellular electron shuttle (17, 22), while Geobacter requires direct contact unless a shuttle is provided (21, 23). P. aerophilum also reduced iron oxide without direct contact (8), but this trait is not universal among Pyrobaculum species. In this study, P. arsenaticum was shown to reduce iron oxides without direct contact; however, P. islandicum and P. calidifontis required direct contact for iron reduction. P. islandicum also requires direct contact for insoluble sulfur reduction. Unlike P. aerophilum, P. islandicum did not grow without direct iron oxide contact, it did not produce any extracellular compounds detectable at 280 nm when grown on iron oxide, and it lacked AHDS oxidase activity. This suggests that these traits in P. aerophilum may be related. The reason for the accumulation of monoribonucleosides only in P. aerophilum Fe(III) oxide hydroxide cell-free supernatants is unknown. The oxidation potential of these compounds (e.g., +1.3 V for adenosine) (9) makes them unlikely electron shuttles. It may be that the putative shuttle gives rise to one of the other LC peaks yet to be analyzed.
As in P. aerophilum, no major and unique membrane protein bands were observed in P. islandicum when it was grown on iron. NADH-dependent ferric reductase activities were significantly higher in P. islandicum cultures grown on iron, suggesting that this activity is regulated and primarily associated with the soluble cellular fraction, as was found for P. aerophilum (8). A 120-kDa membrane protein that was absent from iron- and sulfur-grown cells was observed in thiosulfate-grown cultures. By use of proteomics, the protein was identified as thiosulfate reductase, a heterotrimer with a predicted molybdo-bis-molybdopterin guanine dinucleotide cofactor in the catalytic subunit and multiple iron-sulfur clusters in two subunits. This is the first evidence of this enzyme in either a hyperthermophile or an archaeon, and it appears to be specific for thiosulfate reduction since it was absent from sulfur-grown cultures. A putative heme-containing protein also increased in relative abundance in the membrane fractions of thiosulfate-grown cultures, suggesting that it is related to this metabolism, although the identity and function of this protein are unknown. Therefore, it appears that P. islandicum reduces thiosulfate to sulfide and sulfite on the membrane, and then sulfite is further reduced in the cytoplasm to sulfide by dissimilatory sulfite reductase (19).
Polyheme c-type cytochromes are essential for iron reduction in Shewanella and Geobacter (15, 20). With the exception of P. calidifontis, Pyrobaculum species differ from these bacteria in that Pyrobaculum species do not possess polyheme c-type cytochromes. Based on heme staining, the number of c-type cytochromes decreases from two to one in P. aerophilum when it is shifted from growth on nitrate to growth on iron (8) and from one to none in P. islandicum when shifted from growth on thiosulfate to growth on iron. Therefore, it is likely that c-type cytochromes play little or no role in iron reduction in P. aerophilum and P. islandicum.
In conclusion, dissimilatory iron reduction is ubiquitous in anoxic environments, including geothermal habitats. Our understanding of this process at high temperatures is at a remedial level; however, it appears to be distinct from that found in Shewanella and Geobacter. Type c cytochromes, either polyheme or monoheme, do not appear to be required for iron reduction in Pyrobaculum. While some species can reduce insoluble iron oxide without direct contact, this is not a universal trait among Pyrobaculum species. Differences in iron reduction strategies within a genus have been observed previously in Shewanella (3, 17, 30). Respiratory capabilities vary among and within Pyrobaculum species due to differences in natural histories and the influence of environmental factors, such as pH and reduction potential. Ultimately, understanding these constraints on respiration is necessary in order to model the biogeochemical impacts of these organisms in nature.
Supplementary Material
Acknowledgments
We thank Steve Eyles and the Mass Spectrometry Facility at the University of Massachusetts for their matrix-assisted laser desorption ionization-time of flight analyses for peptide mass fingerprinting. We also thank David Hamilton for his assistance with our growth kinetic experiments.
Footnotes
Published ahead of print on 26 November 2007.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Adams, M. W. W., J. F. Holden, A. L. Menon, G. J. Schut, A. M. Grunden, C. Hou, A. M. Hutchins, F. E. Jenney, Jr., C. Kim, K. Ma, G. Pan, R. Roy, R. Sapra, S. V. Story, and M. F. J. M. Verhagen. 2001. Key role for sulfur in peptide metabolism and in regulation of three hydrogenases in the hyperthermophilic archaeon Pyrococcus furiosus. J. Bacteriol. 183:716-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amo, T., M. L. F. Paje, A. Inagaki, S. Ezaki, H. Atomi, and T. Imanaka. 2002. Pyrobaculum calidifontis sp. nov., a novel hyperthermophilic archaeon that grows in atmospheric air. Archaea 1:113-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beliaev, A. S., D. A. Saffarini, J. L. McLaughlin, and D. Hunnicutt. 2001. MtrC, an outer membrane decahaem c cytochrome required for metal reduction in Shewanella putrefaciens MR-1. Mol. Microbiol. 39:722-730. [DOI] [PubMed] [Google Scholar]
- 4.Boone, D. R., Y. Liu, Z. Zhao, D. L. Balkwill, G. R. Drake, T. O. Stevens, and H. C. Aldrich. 1995. Bacillus infernus sp. nov., an Fe(III)- and Mn(IV)-reducing anaerobe from the deep terrestrial subsurface. Int. J. Syst. Bacteriol. 45:441-448. [DOI] [PubMed] [Google Scholar]
- 5.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 6.Childers, S. E., and D. R. Lovley. 2001. Differences in Fe(III) reduction in the hyperthermophilic archaeon, Pyrobaculum islandicum, versus mesophilic Fe(III)-reducing bacteria. FEMS Microbiol. Lett. 195:253-258. [DOI] [PubMed] [Google Scholar]
- 7.Costilow, R. N. 1981. Biophysical factors in growth, p. 66-78. In P. Gerhardt, R. G. E. Murray, R. N. Costilow, E. W. Nester, W. A. Wood, N. R. Krieg, and G. B. Phillips (ed.), Manual of methods for general bacteriology. American Society for Microbiology, Washington, DC.
- 8.Feinberg, L. F., and J. F. Holden. 2006. Characterization of dissimilatory Fe(III) versus NO3− reduction in the hyperthermophilic archaeon Pyrobaculum aerophilum. J. Bacteriol. 188:525-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goyal, R. N., and A. Sangal. 2002. Electrochemical investigations of adenosine at solid electrodes. J. Electroanal. Chem. 521:72-80. [Google Scholar]
- 10.Huber, R., J. K. Kristjansson, and K. O. Stetter. 1987. Pyrobaculum gen. nov., a new genus of neutrophilic, rod-shaped archaebacteria from continental solfataras growing optimally at 100°C. Arch. Microbiol. 149:95-101. [Google Scholar]
- 11.Huber, R., M. Sacher, A. Vollmann, H. Huber, and D. Rose. 2000. Respiration of arsenate and selenate by hyperthermophilic archaea. Syst. Appl. Microbiol. 23:305-314. [DOI] [PubMed] [Google Scholar]
- 12.Kashefi, K. 1996. Cloning of the nitrate reductase gene from Desulfovibrio desulfuricans (ATCC 27774), an anaerobic sulfate-reducing bacterium. Ph.D. dissertation. University of London, London, England.
- 13.Kashefi, K., and D. R. Lovley. 2000. Reduction of Fe(III), Mn(IV), and toxic metals at 100°C by Pyrobaculum islandicum. Appl. Environ. Microbiol. 66:1050-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kieft, T. L., J. K. Fredrickson, T. C. Onstott, Y. A. Gorby, H. M. Kostandarithes, T. J. Bailey, D. W. Kennedy, S. W. Li, A. E. Plymale, C. M. Spadoni, and M. S. Gray. 1999. Dissimilatory reduction of Fe(III) and other electron acceptors by a Thermus isolate. Appl. Environ. Microbiol. 65:1214-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leang, C., M. V. Coppi, and D. R. Lovley. 2003. OmcB, a c-type polyheme cytochrome, involved in Fe(III) reduction in Geobacter sulfurreducens. J. Bacteriol. 185:2096-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.L'Haridon, S., A.-L. Reysenbach, P. Glénat, D. Prieur, and C. Jeanthon. 1995. Hot subterranean biosphere in a continental oil reservoir. Nature 377:223-224. [PubMed] [Google Scholar]
- 17.Lies, D. P., M. E. Hernandez, A. Kappler, R. E. Mielke, J. A. Gralnick, and D. K. Newman. 2005. Shewanella oneidensis MR-1 uses overlapping pathways for iron reduction at a distance and by direct contact under conditions relevant for biofilms. Appl. Environ. Microbiol. 71:4414-4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu, S. V., J. Zhou, C. Zhang, D. R. Cole, M. Gajdarziska-Josifovska, and T. J. Phelps. 1997. Thermophilic Fe(III)-reducing bacteria from the deep subsurface: the evolutionary implications. Science 277:1106-1109. [Google Scholar]
- 19.Molitor, M., C. Dahl, I. Molitor, U. Schäfer, N. Speich, R. Huber, R. Deutzmann, and H. G. Trüper. 1998. A dissimilatory sirohaem-sulfite-reductase-type protein from the hyperthermophilic archaeon Pyrobaculum islandicum. Microbiology 144:529-541. [DOI] [PubMed] [Google Scholar]
- 20.Myers, C. R., and J. M. Myers. 1997. Cloning and sequencing of cymA, a gene encoding a tetraheme cytochrome c required for reduction of iron(III), fumarate, and nitrate by Shewanella putrefaciens strain MR-1. J. Bacteriol. 179:1143-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nevin, K. P., and D. R. Lovley. 2000. Lack of production of electron-shuttling compounds or solubilization of Fe(III) during reduction of insoluble Fe(III) oxide by Geobacter metallireducens. Appl. Environ. Microbiol. 66:2248-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nevin, K. P., and D. R. Lovley. 2002. Mechanisms for Fe(III) oxide reduction in sedimentary environments. Geomicrobiol. J. 19:141-159. [Google Scholar]
- 23.Reguera, G., K. D. McCarthy, T. Mehta, J. S. Nicoll, M. T. Tuominen, and D. R. Lovley. 2005. Extracellular electron transfer via microbial nanowires. Nature 435:1098-1101. [DOI] [PubMed] [Google Scholar]
- 24.Sako, Y., T. Nunoura, and A. Uchida. 2001. Pyrobaculum oguniense sp. nov., a novel facultatively aerobic and hyperthermophilic archaeon growing at up to 97°C. Int. J. Syst. Evol. Microbiol. 51:303-309. [DOI] [PubMed] [Google Scholar]
- 25.Selig, M., and P. Schönheit. 1994. Oxidation of organic compounds to CO2 with sulfur or thiosulfate as electron acceptor in the anaerobic hyperthermophilic archaea Thermoproteus tenax and Pyrobaculum islandicum proceeds via the citric acid cycle. Arch. Microbiol. 162:286-294. [Google Scholar]
- 26.Shevchenko, A., O. N. Jensen, A. V. Podtelejnikov, F. Sagliocco, M. Wilm, O. Vorm, P. Mortensen, A. Shevchenko, H. Boucherie, and M. Mann. 1996. Linking genome and proteome by mass spectrometry: large-scale identification of yeast proteins from two dimensional gels. Proc. Natl. Acad. Sci. USA 93:14440-14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steen, I. H., H. Hvoslef, T. Lien, and N.-K. Birkeland. 2001. Isocitrate dehydrogenase, malate dehydrogenase, and glutamate dehydrogenase from Archaeoglobus fulgidus. Methods Enzymol. 331:13-26. [DOI] [PubMed] [Google Scholar]
- 28.Takai, K., and K. Horikoshi. 1999. Molecular phylogenetic analysis of archaeal intron-containing genes coding for rRNA obtained from a deep-subsurface geothermal pool. Appl. Environ. Microbiol. 65:5586-5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas, P. E., D. Ryan, and W. Levin. 1976. An improved staining procedure for the detection of the peroxidase activity of cytochrome P-450 on sodium dodecyl sulfate polyacrylamide gels. Anal. Biochem. 75:168-176. [DOI] [PubMed] [Google Scholar]
- 30.Turick, C. E., L. S. Tisa, and F. Caccavo, Jr. 2002. Melanin production and use as a soluble electron shuttle for Fe(III) oxide reduction and as a terminal electron acceptor by Shewanella algae BrY. Appl. Environ. Microbiol. 68:2436-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vargas, M., K. Kashefi, E. L. Blunt-Harris, and D. R. Lovley. 1998. Microbiological evidence for Fe(III) reduction on early Earth. Nature 395:65-67. [DOI] [PubMed] [Google Scholar]
- 32.Völkl, P., R. Huber, E. Drobner, R. Rachel, S. Burggraf, A. Trincone, and K. O. Stetter. 1993. Pyrobaculum aerophilum sp. nov., a novel nitrate-reducing hyperthermophilic archaeum. Appl. Environ. Microbiol. 59:2918-2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitman, W. B., D. C. Coleman, and W. J. Wiebe. 1998. Prokaryotes: the unseen majority. Proc. Natl. Acad. Sci. USA 95:6578-6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yennaco, L. J., Y. Hu, and J. F. Holden. 2007. Characterization of malate dehydrogenase from the hyperthermophilic archaeon Pyrobaculum islandicum. Extremophiles 11:741-746. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.