Abstract
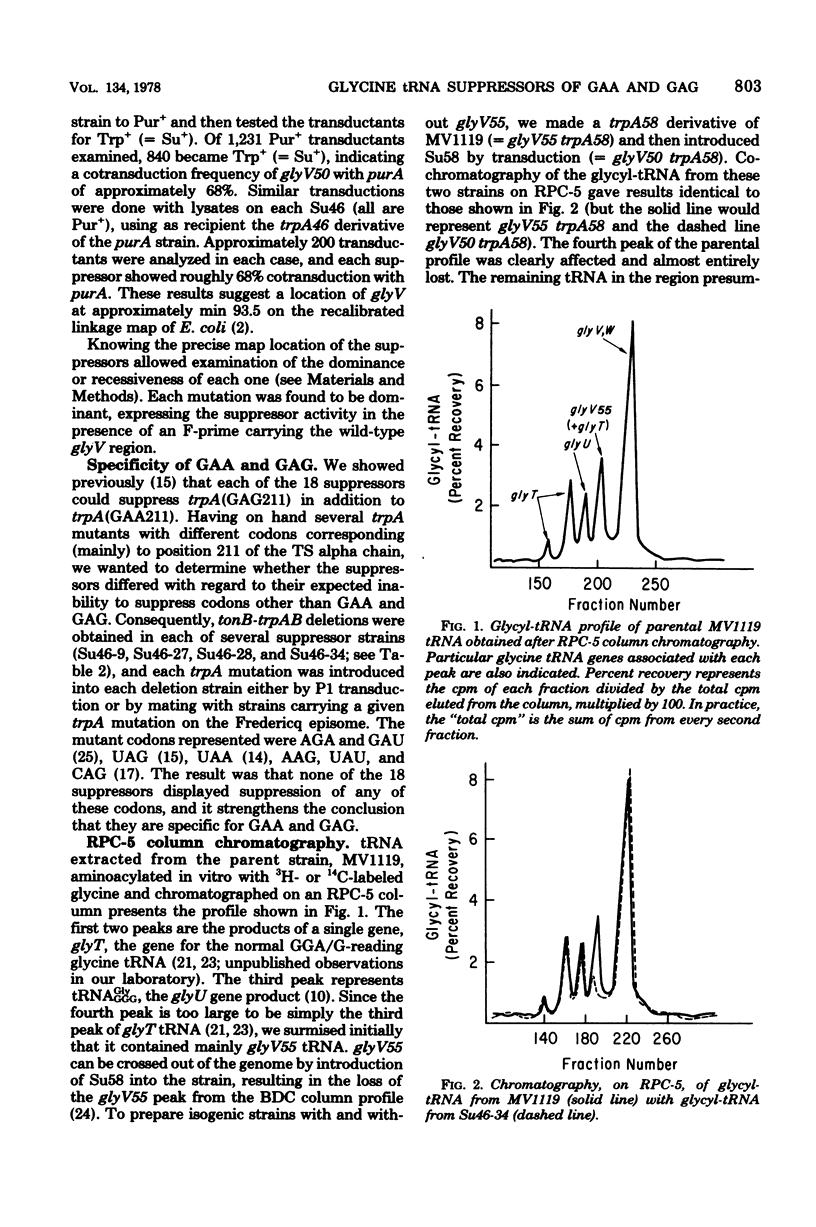
Glutamic acid codon suppressors in 18 isogenic strains of Escherichia coli have been further characterized as to map location, dominance, growth rates in various media, suppression of the GAG codon, and tRNA profiles after reversed-phase column chromatography. In general the evidence supports the conclusion that all of these suppressors are due to mutations in glyV55, the gene for a GGA/G-reading mutant form of glyV tRNA, and that they represent several different classes that may correspond to at least as many different nucleotide changes. Furthermore, 17 of the 18 suppressors can coexist in a haploid genome with a glyT suppressor that is devoid of GGA-reading ability. This result indicates the retention by those glyV suppressors of some ability to respond to GGA as well as the acquisition of the ability to read GAA, and suggests the possibility of "wobble" in the middle position of the anticodons of those tRNA's.
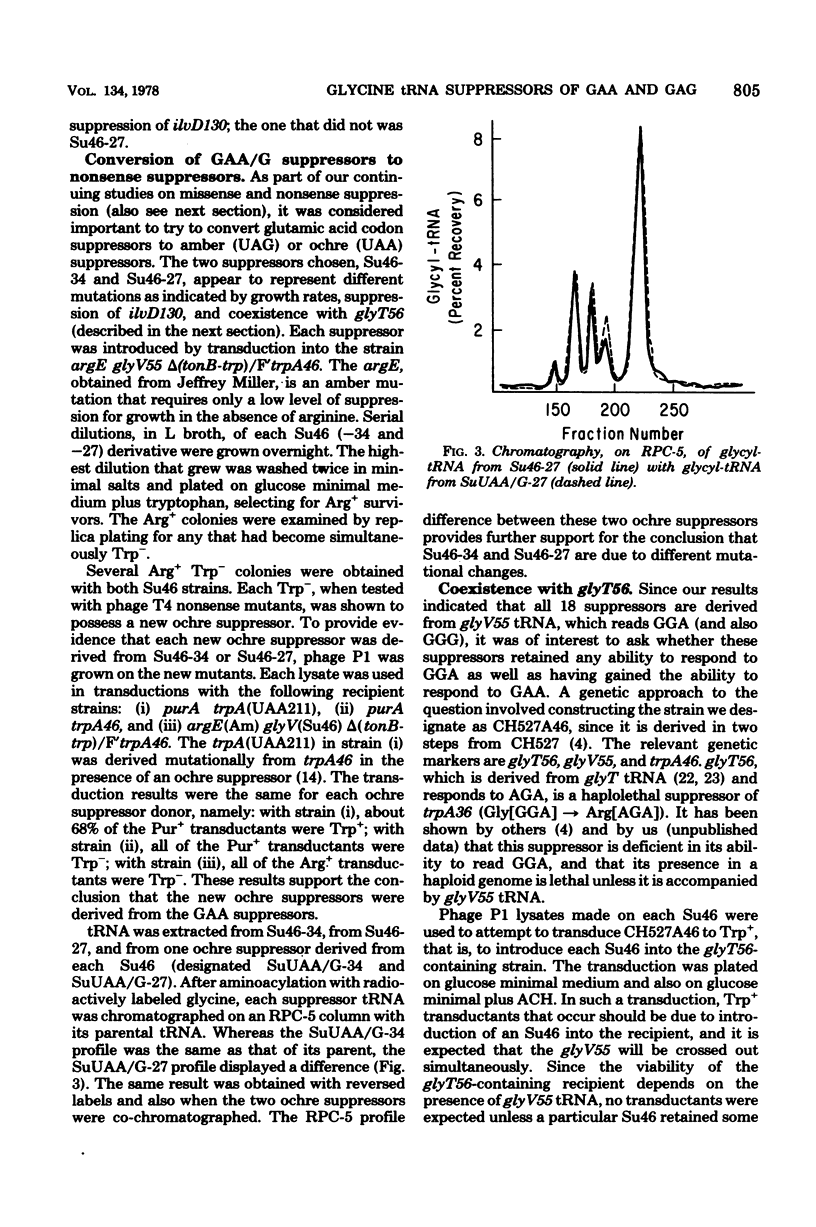
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN M. K., YANOFSKY C. A BIOCHEMICAL AND GENETIC STUDY OF REVERSION WITH THE A-GENE A-PROTEIN SYSTEM OF ESCHERICHIA COLI TRYPTOPHAN SYNTHETASE. Genetics. 1963 Aug;48:1065–1083. doi: 10.1093/genetics/48.8.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon J., Curry J. B. Genetically and chemically derived missense suppressor transfer RNA's with altered enzymic aminoacylation rates. J Mol Biol. 1968 Dec 14;38(2):201–216. doi: 10.1016/0022-2836(68)90406-3. [DOI] [PubMed] [Google Scholar]
- Carbon J., Squires C., Hill C. W. Glycine transfer RNA of Escherichia coli. II. Impaired GGA-recognition in strains containing a genetically altered transfer RNA; reversal by a secondary suppressor mutation. J Mol Biol. 1970 Sep 28;52(3):571–584. doi: 10.1016/0022-2836(70)90420-1. [DOI] [PubMed] [Google Scholar]
- Coukell M. B., Yanofsky C. Influence of chromosome structure on the frequency of tonB trp deletions in Escherichia coli. J Bacteriol. 1971 Mar;105(3):864–872. doi: 10.1128/jb.105.3.864-872.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F. H. Codon--anticodon pairing: the wobble hypothesis. J Mol Biol. 1966 Aug;19(2):548–555. doi: 10.1016/s0022-2836(66)80022-0. [DOI] [PubMed] [Google Scholar]
- Fleck E. W., Carbon J. Multiple gene loci for a single species of glycine transfer ribonucleic acid. J Bacteriol. 1975 May;122(2):492–501. doi: 10.1128/jb.122.2.492-501.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUEST J. R., YANOFSKY C. MUTATIONALLY INDUCED AMINO ACID SUBSTITUTIONS IN A TRYPTIC PEPTIDE OF THE TRYPTOPHAN SYNTHETASE A PROTEIN. J Biol Chem. 1965 Feb;240:679–689. [PubMed] [Google Scholar]
- Hill C. W., Combriato G., Dolph W. Three different missense suppressor mutations affecting the tRNA GGG Gly species of Escherichia coli. J Bacteriol. 1974 Feb;117(2):351–359. doi: 10.1128/jb.117.2.351-359.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh D. Tryptophan transfer RNA as the UGA suppressor. J Mol Biol. 1971 Jun 14;58(2):439–458. doi: 10.1016/0022-2836(71)90362-7. [DOI] [PubMed] [Google Scholar]
- Kurland C. G., Rigler R., Ehrenberg M., Blomberg C. Allosteric mechanism for codon-dependent tRNA selection on ribosomes. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4248–4251. doi: 10.1073/pnas.72.11.4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgola E. J., Yanofsky C. Selection for new amino acids at position 211 of the tryptophan synthetase alpha chain of Escherichia coli. J Mol Biol. 1974 Jul 15;86(4):775–784. doi: 10.1016/0022-2836(74)90353-2. [DOI] [PubMed] [Google Scholar]
- Murgola E. J., Yanofsky C. Structural interactions between amino acid residues at positions 22 and 211 in the tryptophan synthetase alpha chain of Escherichia coli. J Bacteriol. 1974 Feb;117(2):444–448. doi: 10.1128/jb.117.2.444-448.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgola E. J., Yanofsky C. Suppression of glutamic acid codons by mutant glycine transfer ribonucleic acid. J Bacteriol. 1974 Feb;117(2):439–443. doi: 10.1128/jb.117.2.439-443.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrem D. L., Berg P. Glycyl transfer ribonucleic acid synthetase from Escherichia coli: purification, properties, and substrate binding. Biochemistry. 1974 Mar 26;13(7):1338–1348. doi: 10.1021/bi00704a006. [DOI] [PubMed] [Google Scholar]
- Pearson R. L., Weiss J. F., Kelmers A. D. Improved separation of transfer RNA's on polychlorotrifuoroethylene-supported reversed-phase chromatography columns. Biochim Biophys Acta. 1971 Feb 11;228(3):770–774. doi: 10.1016/0005-2787(71)90748-9. [DOI] [PubMed] [Google Scholar]
- Reid P., Berg P. T4 bacteriophage mutants suppressible by a missense suppressor which inserts glycine in place of arginine for the codon AGA. J Virol. 1968 Sep;2(9):905–914. doi: 10.1128/jvi.2.9.905-914.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle D. L., Carbon J. Frameshift suppression: a nucleotide addition in the anticodon of a glycine transfer RNA. Nat New Biol. 1973 Apr 25;242(121):230–234. doi: 10.1038/newbio242230a0. [DOI] [PubMed] [Google Scholar]
- Roberts J. W., Carbon J. Molecular mechanism for missense suppression in E. coli. Nature. 1974 Aug 2;250(465):412–414. doi: 10.1038/250412a0. [DOI] [PubMed] [Google Scholar]
- Roberts J. W., Carbon J. Nucleotide sequence studies of normal and genetically altered glycine transfer ribonucleic acids from Escherichia coli. J Biol Chem. 1975 Jul 25;250(14):5530–5541. [PubMed] [Google Scholar]
- Squires C., Carbon J. Normal and mutant glycine transfer RNAs. Nat New Biol. 1971 Oct 27;233(43):274–277. doi: 10.1038/newbio233274a0. [DOI] [PubMed] [Google Scholar]
- Yanofsky C., Ito J., Horn V. Amino acid replacements and the genetic code. Cold Spring Harb Symp Quant Biol. 1966;31:151–162. doi: 10.1101/sqb.1966.031.01.023. [DOI] [PubMed] [Google Scholar]


