Abstract
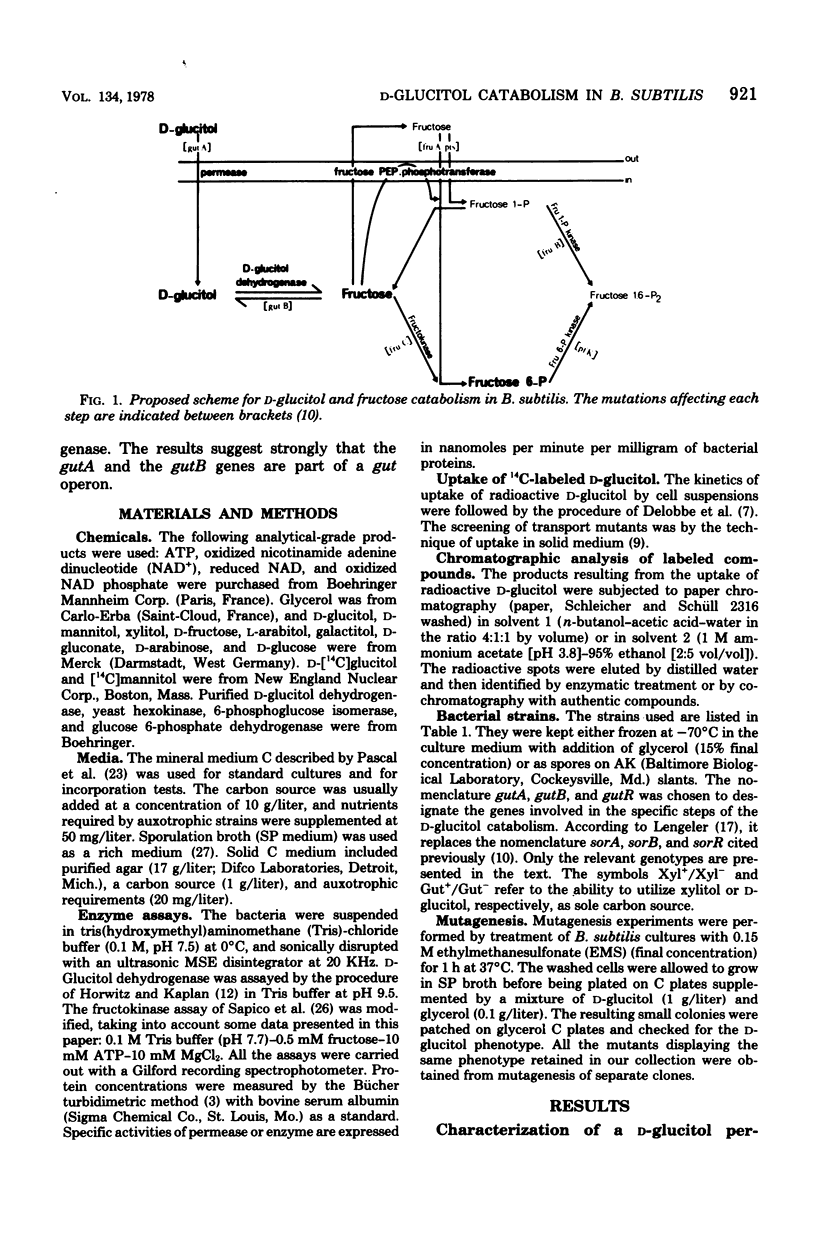
The catabolic pathway of D-glucitol (sorbitol) in Bacillus subtilis Marburg 168M is characterized. It includes (i) a transport step catalyzed by a D-glucitol permease which is affected by the gutA mutations, (ii) an oxidation step of the intracellular D-glucitol catalyzed by a D-glucitol dehydrogenase, generating intracellular fructose, affected by gutB mutations, and (iii) phosphorylation of the intracellular fructose either at the C1 site or at the C6 site as described previously (A. Delobbe et al., Eur. J. Biochem., 66:485-491, 1976; A. Delobbe et al., EUR. J. Biochem. 51:503-510, 1975). Additional data are given concerning the phosphorylation of fructose by a fructokinase (fructose ATP 6-phosphotransferase), which is affected by the fruC mutation. The isolation of regulatory mutants affected in gutR that synthesize constitutively both the permease and the dehydrogenase indicates the existence of a D-glucitol operon in B. subtilis. Unlike the wild-type strain, these mutants are able to utilize D-xylitol as sole carbon source.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony R. S., Spector L. B. A phosphoenzyme intermediary in acetate kinase action. J Biol Chem. 1970 Dec 25;245(24):6739–6741. [PubMed] [Google Scholar]
- Ballard F. J. Kinetic studies with liver galactokinase. Biochem J. 1966 Oct;101(1):70–75. doi: 10.1042/bj1010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delobbe A., Chalumeau H., Claverie J. M., Gay P. Phosphorylation of intracellular fructose in Bacillus subtilis mediated by phosphoenolpyruvate-1-fructose phosphotransferase. Eur J Biochem. 1976 Jul 15;66(3):485–491. doi: 10.1111/j.1432-1033.1976.tb10573.x. [DOI] [PubMed] [Google Scholar]
- Delobbe A., Chalumeau H., Gay P. Existence of two alternative pathways for fructose and sorbitol metabolism in Bacillus subtilis Marburg. Eur J Biochem. 1975 Feb 21;51(2):503–510. doi: 10.1111/j.1432-1033.1975.tb03950.x. [DOI] [PubMed] [Google Scholar]
- Delobbe A., Haguenauer R., Rapoport G. Studies on the transport of -methyl-D-glucoside in Bacillus subtilis 168. Biochimie. 1971;53(9):1015–1021. doi: 10.1016/s0300-9084(71)80069-x. [DOI] [PubMed] [Google Scholar]
- Gay P., Cordier P., Marquet M., Delobbe A. Carbohydrate metabolism and transport in Bacillus subtilis. A study of ctr mutations. Mol Gen Genet. 1973 Mar 19;121(4):355–368. doi: 10.1007/BF00433234. [DOI] [PubMed] [Google Scholar]
- Gay P., Delobbe A. Fructose transport in Bacillus subtilis. Eur J Biochem. 1977 Oct 3;79(2):363–373. doi: 10.1111/j.1432-1033.1977.tb11817.x. [DOI] [PubMed] [Google Scholar]
- Gay P., Rapoport G. Etude des mutants dépourvus de fructose-1-phosphate kinase chez Bacillus subtilis. C R Acad Sci Hebd Seances Acad Sci D. 1970 Jul 20;271(3):374–377. [PubMed] [Google Scholar]
- HORWITZ S. B., KAPLAN N. O. HEXITOL DEHYDROGENASES OF BACILLUS SUBTILIS. J Biol Chem. 1964 Mar;239:830–838. [PubMed] [Google Scholar]
- KEPES A., MONOD J. Etude du fonctionnement de la galactoside-perméase d'Escherichia coli. C R Hebd Seances Acad Sci. 1957 Feb 4;244(6):809–811. [PubMed] [Google Scholar]
- Kelker N. E., Hanson T. E., Anderson R. L. Alternate pathways of D-fructose metabolism in Aerobacter aerogenes. A specific D-fructokinase and its preferential role in the metabolism of sucrose. J Biol Chem. 1970 Apr 25;245(8):2060–2065. [PubMed] [Google Scholar]
- Kepes A. Trois classes de systèmes de transport chez les bactéries. Biochimie. 1973;55(6):693–702. [PubMed] [Google Scholar]
- Lengeler J., Lin E. C. Reversal of the mannitol-sorbitol diauxie in Escherichia coli. J Bacteriol. 1972 Nov;112(2):840–848. doi: 10.1128/jb.112.2.840-848.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengeler J. Mutations affecting transport of the hexitols D-mannitol, D-glucitol, and galactitol in Escherichia coli K-12: isolation and mapping. J Bacteriol. 1975 Oct;124(1):26–38. doi: 10.1128/jb.124.1.26-38.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengeler J. Nature and properties of hexitol transport systems in Escherichia coli. J Bacteriol. 1975 Oct;124(1):39–47. doi: 10.1128/jb.124.1.39-47.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepesant J. A., Kunst F., Lepesant-Kejzlarová J., Dedonder R. Chromosomal location of mutations affecting sucrose metabolism in Bacillus subtilis Marburg. Mol Gen Genet. 1972;118(2):135–160. doi: 10.1007/BF00267084. [DOI] [PubMed] [Google Scholar]
- Niaudet B., Gay P., Dedonder R. Identification of the structural gene of the PEP-phosphotransferase enzyme I in Bacillus subtilis Marburg. Mol Gen Genet. 1975;136(4):337–349. doi: 10.1007/BF00341718. [DOI] [PubMed] [Google Scholar]
- Pascal M., Kunst F., Lepesant J. A., Dedonder R. Characterization of two sucrase activities in Bacillus subtilis Marburg. Biochimie. 1971;53(10):1059–1066. doi: 10.1016/s0300-9084(71)80193-1. [DOI] [PubMed] [Google Scholar]
- SCOLNICK E. M., LIN E. C. Parallel induction of D-arabitol and D-sorbitol dehydrogenases. J Bacteriol. 1962 Oct;84:631–637. doi: 10.1128/jb.84.4.631-637.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Simoni R. D., Roseman S. The physiological behavior of enzyme I and heat-stable protein mutants of a bacterial phosphotransferase system. J Biol Chem. 1970 Nov 10;245(21):5870–5873. [PubMed] [Google Scholar]
- Sapico V., Hanson T. E., Walter R. W., Anderson R. L. Metabolism of D-fructose in Aerobacter aerogenes: analysis of mutants lacking D-fructose 6-phosphate kinase and D-fructose 1,6-diphosphatase. J Bacteriol. 1968 Jul;96(1):51–54. doi: 10.1128/jb.96.1.51-54.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon E., Lin E. C. Mutations affecting the dissimilation of mannitol by Escherichia coli K-12. J Bacteriol. 1972 Aug;111(2):566–574. doi: 10.1128/jb.111.2.566-574.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Lin E. C. Two classes of pleiotropic mutants of Aerobacter aerogenes lacking components of a phosphoenolpyruvate-dependent phosphotransferase system. Proc Natl Acad Sci U S A. 1967 Apr;57(4):913–919. doi: 10.1073/pnas.57.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C. T., Jr, Spector L. B. A phosphoenzyme intermediary in phosphoglycerate kinase action. J Biol Chem. 1971 Mar 10;246(5):1255–1261. [PubMed] [Google Scholar]
- Willecke K., Pardee A. B. Inducible transport of citrate in a Gram-positive bacterium, Bacillus subtilis. J Biol Chem. 1971 Feb 25;246(4):1032–1040. [PubMed] [Google Scholar]


