Abstract
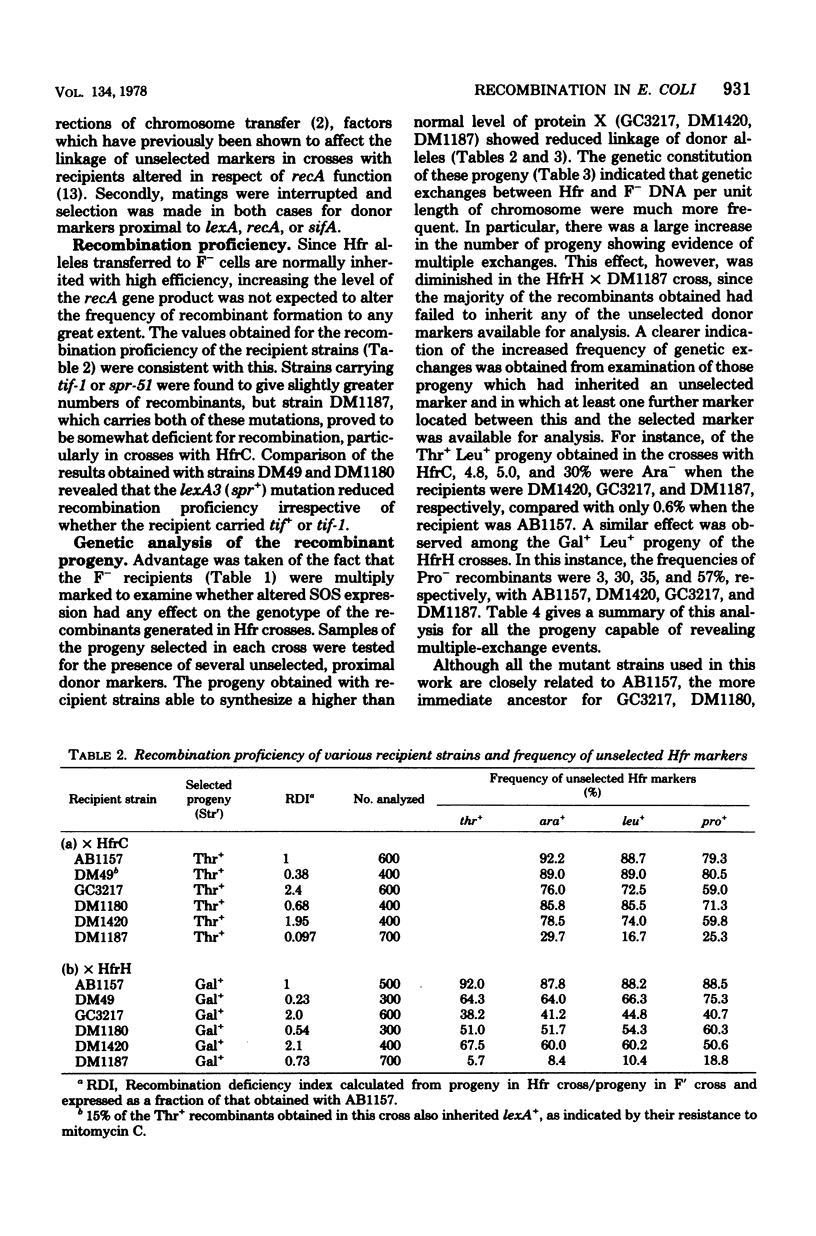
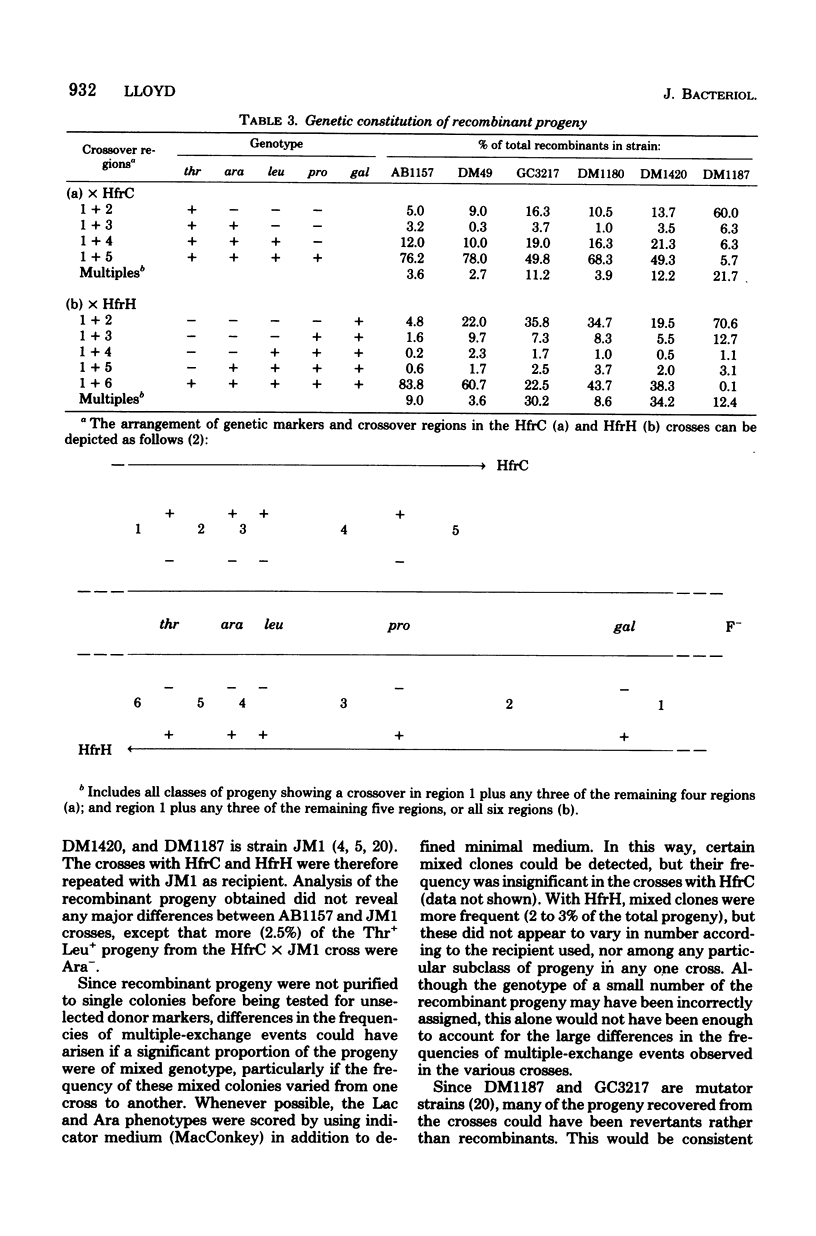
Genetic recombination was studied in Escherichia coli F- strains in which synthesis of the recA gene product protein X is increased due to mutation in either recA (tif-1) or lexA (spr). When a single donor marker was selected, the recombination proficiency of these strains was not significantly altered in Hfr crosses. However, linkage of unselected, proximal Hfr markers was found to be much reduced among the progeny tested, and more of the progeny showed evidence of multiple exchanges between donor and recipient DNA. These effects were much more apparent when the recipient carried both tif-1 and spr mutations, but in this case recombination proficiency was reduced compared with those strains carrying either mutation alone, particularly in crosses with Hfr Cavalli. A lexA mutation was found to suppress the effect of tif-1 on the recombinant genotype.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S. K., Oishi M. Factor which affects the mode of genetic recombination in E. Coli. Nature. 1975 Jan 10;253(5487):138–140. doi: 10.1038/253138a0. [DOI] [PubMed] [Google Scholar]
- CLARK A. J., MARGULIES A. D. ISOLATION AND CHARACTERIZATION OF RECOMBINATION-DEFICIENT MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1965 Feb;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellazzi M., George J., Buttin G. [Prophage induction and cell division in E. coli. II. Linked (recA, zab) and unlinked (lex) suppressors of tif-1-mediated induction and filamentation]. Mol Gen Genet. 1972;119(2):153–174. doi: 10.1007/BF00269134. [DOI] [PubMed] [Google Scholar]
- Castellazzi M., Morand P., George J., Buttin G. Prophage induction and cell division in E. coli. V. Dominance and complementation analysis in partial diploids with pleiotropic mutations (tif, recA, zab and lexB) at the recA locus. Mol Gen Genet. 1977 Jun 24;153(3):297–310. doi: 10.1007/BF00431595. [DOI] [PubMed] [Google Scholar]
- Clark A. J. Recombination deficient mutants of E. coli and other bacteria. Annu Rev Genet. 1973;7:67–86. doi: 10.1146/annurev.ge.07.120173.000435. [DOI] [PubMed] [Google Scholar]
- Defais M., Caillet-Fauquet P., Fox M. S., Radman M. Induction kinetics of mutagenic DNA repair activity in E. coli following ultraviolet irradiation. Mol Gen Genet. 1976 Oct 18;148(2):125–130. doi: 10.1007/BF00268375. [DOI] [PubMed] [Google Scholar]
- Emmerson P. T., West S. C. Identification of protein X of Escherichia coli as the recA+/tif+ gene product. Mol Gen Genet. 1977 Sep 21;155(1):77–85. doi: 10.1007/BF00268563. [DOI] [PubMed] [Google Scholar]
- George J., Castellazzi M., Buttin G. Prophage induction and cell division in E. coli. III. Mutations sfiA and sfiB restore division in tif and lon strains and permit the expression of mutator properties of tif. Mol Gen Genet. 1975 Oct 22;140(4):309–332. [PubMed] [Google Scholar]
- Gudas L. J., Pardee A. B. Model for regulation of Escherichia coli DNA repair functions. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2330–2334. doi: 10.1073/pnas.72.6.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDERBERG J., LEDERBERG E. M. Replica plating and indirect selection of bacterial mutants. J Bacteriol. 1952 Mar;63(3):399–406. doi: 10.1128/jb.63.3.399-406.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOW B., WOOD T. H. A QUICK AND EFFICIENT METHOD FOR INTERRUPTION OF BACTERIAL CONJUGATION. Genet Res. 1965 Jul;6:300–303. doi: 10.1017/s001667230000416x. [DOI] [PubMed] [Google Scholar]
- Lloyd R. G., Low B. Some genetic consequences of changes in the level of recA gene function in Escherichia coli K-12. Genetics. 1976 Dec;84(4):675–695. doi: 10.1093/genetics/84.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. Rapid mapping of conditional and auxotrophic mutations in Escherichia coli K-12. J Bacteriol. 1973 Feb;113(2):798–812. doi: 10.1128/jb.113.2.798-812.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan S. K., Datta A. R. Nature of recombinants produced by the RecBC and the RecF pathways in Escherichia coli. Nature. 1977 Apr 14;266(5603):652–653. doi: 10.1038/266652a0. [DOI] [PubMed] [Google Scholar]
- McEntee K., Hesse J. E., Epstein W. Identification and radiochemical purification of the recA protein of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3979–3983. doi: 10.1073/pnas.73.11.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morand P., Blanco M., Devoret R. Characterization of lexB mutations in Escherichia coli K-12. J Bacteriol. 1977 Aug;131(2):572–582. doi: 10.1128/jb.131.2.572-582.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A., Guild W. R. Activity of deoxyribonucleic acid fragments of defined size in Bacillus subtilis transformation. J Bacteriol. 1972 Oct;112(1):220–223. doi: 10.1128/jb.112.1.220-223.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mound D. W., Walker A. C., Kosel C. Suppression of lex mutations affecting deoxyribonucleic acid repair in Escherichia coli K-12 by closely linked thermosensitive mutations. J Bacteriol. 1973 Nov;116(2):950–956. doi: 10.1128/jb.116.2.950-956.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount D. W. A mutant of Escherichia coli showing constitutive expression of the lysogenic induction and error-prone DNA repair pathways. Proc Natl Acad Sci U S A. 1977 Jan;74(1):300–304. doi: 10.1073/pnas.74.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount D. W., Kosel C. K., Walker A. Inducible, error-free DNA Repair in tsl recA mutants of E. coli. Mol Gen Genet. 1976 Jul 5;146(1):37–41. doi: 10.1007/BF00267980. [DOI] [PubMed] [Google Scholar]
- Mount D. W., Low K. B., Edmiston S. J. Dominant mutations (lex) in Escherichia coli K-12 which affect radiation sensitivity and frequency of ultraviolet lght-induced mutations. J Bacteriol. 1972 Nov;112(2):886–893. doi: 10.1128/jb.112.2.886-893.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radman M. SOS repair hypothesis: phenomenology of an inducible DNA repair which is accompanied by mutagenesis. Basic Life Sci. 1975;5A:355–367. doi: 10.1007/978-1-4684-2895-7_48. [DOI] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W., Mount D. W. Inactivation and proteolytic cleavage of phage lambda repressor in vitro in an ATP-dependent reaction. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2283–2287. doi: 10.1073/pnas.74.6.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Thermal enhancement of ultraviolet mutability in a tif-1 uvrA derivative of Escherichia coli B-r: evidence that ultraviolet mutagenesis depends upon an inducible function. Proc Natl Acad Sci U S A. 1974 May;71(5):1930–1934. doi: 10.1073/pnas.71.5.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


