Abstract
Nuclear factor κB (NF-κB) plays a key regulatory role in host cell responses to Helicobacter pylori infection in humans. Although mice are routinely used as a model to study H. pylori pathogenesis, the role of NF-κB in murine cell responses to helicobacters has not been studied in detail. We thus investigated the abilities of different Helicobacter isolates to induce NF-κB-dependent responses in murine gastric epithelial cells (GECs) and in transgenic mice harboring an NF-κB-responsive lacZ reporter gene. H. pylori and Helicobacter felis strains up-regulated the synthesis in mouse GECs of the NF-κB-dependent chemokines KC (CXCL1) and MIP-2 (CXCL2). These responses were cag pathogenicity island (cagPAI) independent and could be abolished by pretreatment with a pharmacological inhibitor of NF-κB. Consistent with the in vitro data, experimental Helicobacter infection of transgenic mice resulted in increased numbers of GECs with nuclear β-galactosidase activity, which is indicative of specific NF-κB activation. The numbers of β-galactosidase-positive cells in mice were significantly increased at day 1 postinoculation with wild-type H. pylori strains harboring or not harboring a functional cagPAI, compared to naive animals (P = 0.007 and P = 0.04, respectively). Strikingly, however, no differences were observed in the levels of gastric NF-κB activation at day 1 postinoculation with H. felis or at day 30 or 135 postinoculation with H. pylori. This work demonstrates for the first time the induction of NF-κB activation within gastric mucosal cells during acute H. pylori infection. Furthermore, the data suggest that helicobacters may be able to regulate NF-κB signaling during chronic infection.
Gastritis is a major characteristic of the disease process initiated by the bacterial pathogen Helicobacter pylori. Recruitment of inflammatory cell populations to H. pylori-infected gastric mucosa is mediated by locally synthesized factors. As H. pylori bacteria colonize the external surfaces of the gastric mucosa, it is likely that epithelial cells lining the mucosa are the major sources of the proinflammatory factors produced during infection.
H. pylori has been shown to up-regulate the expression of the transcription factor nuclear factor κB (NF-κB) in gastric epithelial cells (GECs) in vitro (21), as well as during chronic infection in humans (17, 46). NF-κB is a key regulator of proinflammatory responses in host cells (15). This transcription factor is ubiquitously present in all types of eukaryotic cells, where it exists in an inactive form, as either a hetero- or a homodimeric molecule. Activation of the NF-κB pathway by proinflammatory stimuli, such as live microorganisms or their products, results in the release of the inactive dimers from inhibitors of the NF-κB family (IκB). In the case of the classical NF-κB signaling pathway, these activated dimers are generally composed of p50 and p65 subunits, which translocate to the nucleus, thereby up-regulating the expression of various target genes (15). Many of these genes encode factors with immune or inflammatory functions.
H. pylori strains that harbor a cag pathogenicity island (cagPAI) are able to specifically induce rapid NF-κB activation in epithelial cells in vitro (4), culminating in the production of immune mediators such as interleukin-8 (IL-8) (21, 37), monocyte chemoattractant protein 1 (MCP-1) (24), Gro-α (16), and intercellular adhesion molecule 1 (25). The cagPAI encodes a bacterial type IV secretion capable of translocating effector molecules that initiate an inflammatory signaling cascade involving NF-κB in host cells (3, 48). cagPAI-positive H. pylori strains were found to be more frequently associated with severe polymorphoneutrophil (PMN) infiltration of the gastric mucosa (5, 30, 52), which is consistent with the known role of IL-8 in PMN cell recruitment and activation.
Mice experimentally infected with H. pylori develop gastritis, albeit of less severity than that observed in human H. pylori infection (12, 35, 47). The absence of a murine homologue of IL-8 is one factor that is often cited to explain the reduced levels of inflammation in this model. Mongolian gerbils, however, also lack IL-8 yet develop very severe gastric lesions in response to H. pylori infection (33, 34, 38). Moreover, the fact that experimental infection of mice with the related gastric species Helicobacter felis results in severe gastritis (35) suggests that bacterial factors may be important.
Although the biological equivalents of human IL-8 for inflammatory responses in rodent H. pylori models have yet to be defined, the murine CXC chemokines KC (CXCL1) and MIP-2 (CXCL2) seem likely candidates. These chemokines have biological functions analogous to those of human IL-8 (29, 32) and, in common with the latter, are regulated by NF-κB (44, 50). Furthermore, gene expression levels of KC (14, 27, 51) and MIP-2 (14, 27) were found to be increased in the gastric mucosa of H. pylori-infected mice. Taken together, it seemed that KC and MIP-2 may promote leukocyte recruitment in mice infected with H. pylori.
To gain further insight into host cell responses in Helicobacter infection models, we investigated the ability of Helicobacter isolates to promote CXC chemokine synthesis in murine GECs in vitro. For this purpose, we chose the GSM06 cell line, which exhibits many of the features of normal gastric surface epithelial cells (8, 41-43). The responses of mouse host cells to Helicobacter infection were further investigated in vivo by using transgenic mice possessing an NF-κB-responsive lacZ reporter gene (20, 36). Gastric mucosal levels of NF-κB activation in the mice were measured in response to infection with mouse-colonizing H. pylori and H. felis strains. Thus, for the first time, we have been able to demonstrate and quantify NF-κB responses within the gastric mucosa during an acute Helicobacter infection. This study highlights the differences in host-pathogen interactions in models of Helicobacter-induced inflammation.
MATERIALS AND METHODS
Mice.
Transgenic κB-lacZ mice (p105-lacZ line 189-4) on a C57BL/6J × SJL mixed background (20, 36) were rederived by embryo transfer to be Helicobacter free. The Helicobacter status of animals was confirmed by enzyme-linked immunosorbent assay (ELISA) of serum antibodies and by culturing of the intestinal and cecal homogenates for intestinal Helicobacter spp. by the techniques described below. Animals were housed in polycarbonate cages in isolators and fed a commercial pellet diet with water ad libitum. Animal handling and experimentation were performed in accordance with institutional guidelines and current French legislation (law 87-848).
Bacterial strains and culture conditions.
Studies were performed with parental H. pylori strains 245 (31), SS1 (12), and 251 (31); H. felis strain CS1 (ATCC 49179) (13); and a cagM mutant of H. pylori 251 (48). The H. pylori 251 cagM mutant strain was constructed by gene inactivation with a construct derived from mini-Tn3-Km mutagenesis of a library of cloned H. pylori genomic DNA (19). The transposon insertion site was mapped to nucleotide position 935 in the cagM gene of H. pylori reference strain 26695 (45).
Bacteria were routinely subcultured on Blood Agar Base No. 2 (Oxoid, Basingstoke, United Kingdom) medium supplemented with 10% (vol/vol) horse blood (bioMérieux, Marcy-l'Étoile, France) and antibiotics (12). Plates were incubated under microaerobic conditions at 37°C. Broth cultures were prepared in 10 ml brain heart infusion broth (Oxoid) containing 10% (vol/vol) heat-inactivated fetal calf serum (FCS; Invitrogen Life Technologies, Cergy-Pontoise, France). Cultures were incubated for 16 to 20 h with shaking in tissue culture flasks (Falcon; Becton Dickinson Labware, Le Pont de Claix, France) under microaerobic conditions at 37°C. Bacteria were washed twice in phosphate-buffered saline (PBS; pH 7.4) prior to resuspension in tissue culture medium. Viable counts were performed on bacterial suspensions by serial dilution in sterile peptone trypsin broth (12).
Cell coculture assays.
The GSMO6 mouse GEC line was established from transgenic mice harboring a temperature-sensitive simian virus 40 large T antigen gene and was grown as described previously (41, 42). Briefly, the cells were routinely cultured in Dulbecco's modified Eagle medium-nutrient mixture F-12 supplemented with 10% FCS, 1% (vol/vol) insulin-transferrin-selenite, 10 ng/ml epidermal growth factor, 100 IU/ml penicillin, and 100 mg/liter streptomycin (all reagents were from Invitrogen). Diluted cell suspensions were seeded onto collagen-coated (2 mg/ml rat tail collagen type 1; Sigma Chemical Co., St. Louis, MO) culture plates and incubated in 5% CO2. The cells were routinely grown at the permissive temperature of 33°C and moved to 37°C on the evening prior to experiments. The AGS cell line was grown routinely in RPMI 1640 medium containing 10% FCS and supplemented with penicillin-streptomycin and 10 mM l-glutamine (Invitrogen) at 37°C in 5% CO2 (31).
Tissue culture cells that had been serum starved overnight were washed three times, and the appropriate medium was replaced with medium containing Helicobacter bacteria at a multiplicity of infection of 1:10 to 1:100 (31). As positive controls, AGS cells were treated with 10 ng/ml human tumor necrosis factor (R&D Systems, Minneapolis, MN). Plates of nontreated or stimulated cell lines were incubated at 37°C in 5% CO2 for the required times. Proteasome inhibition studies were performed by pretreatment of cells with 50 μM N-acetyl-leucinyl-leucinyl-norleucinal-H (ALLN). The cells were washed twice before addition of bacteria. Cell toxicity was determined by measurement of released lactate dehydrogenase with the Roche cytotoxicity detection kit (Roche Diagnostics, Meylan, France).
Electrophoretic mobility shift assay (EMSA).
Nuclear extracts were prepared from cells (at 2 to 4 h poststimulation) as described previously (31). Five micrograms of nuclear extract was combined with 32P-labeled DNA probe corresponding to the κB site located in the major histocompatibility complex class Ib gene promoter and then run on 5% (wt/vol) polyacrylamide gels in Tris-borate-EDTA buffer (36).
Cell transfection assays.
AGS cells were plated in 24-well plates at a density of 8 × 104 cells per well and transfected on the following day with FuGene6 reagent (Roche Diagnostics) (31) with an immunoglobulin (κ)-luciferase reporter construct (36). Cotransfection with 0.3 μg of a β-galactosidase reporter plasmid (Invitrogen) was used to normalize transfection efficiencies. Following transfection, cells were cocultured for 6 h with bacteria. Lysed cells were assayed for luciferase activities with a 96-well luminometer (Berthold France SA, Thoiry, France).
Mouse colonization studies.
Mice (male or female; 6 to 12 weeks old) were inoculated intragastrically with bacterial suspensions which had been prepared directly in peptone trypsin broth from 36-h plate cultures. Each mouse was administered an aliquot (0.1 ml) of the suspension containing approximately 1 × 108 to 5 × 108 CFU/ml with a Teflon catheter as described previously (12, 13). The presence of gastric Helicobacter infection in mice was determined by quantitative culture of gastric tissue fragments containing both the antrum and corpus and by ELISA of serum antibodies (11). Alternatively, a portion of these fragments was placed in PBS containing protease inhibitors (Roche) and frozen at −20°C prior to analysis by cytokine ELISA (see below). The remainder of the stomach tissues were cut into transversal sections (each approximately 2 mm wide, 1 cm long) comprising tissue from the antrum, body, fundus, and cardia. Tissue sections were processed for Harris' hematoxylin-eosin staining and/or β-galactosidase activity detection (1). For the latter, tissues were fixed in ethanol for 3 h, dehydrated three times (30 min each) in xylene, and then embedded by three passages (30 min each) at 44°C in low-melting-point paraffin (Merck, VWR, Fontenay-sous-Bois, France).
Histological studies.
Formalin-fixed sections were assessed blind (by M.R.H.) for pathological changes by using a scoring scheme based on that of Eaton et al. (9). Inflammatory scores for numbers of PMNs and lymphocyte or plasma cell infiltration in the lamina propria of the antrum and fundus were graded as follows: 1, mild multifocal (scattered clumps of two or three cells); 2, mild widespread (widespread scattering of cells across most of the region, e.g., antrum or fundus) or moderate multifocal (larger clumps of cells seen in a few fields per region); 3, mild widespread and moderate multifocal or severe multifocal (large infiltration of cells across whole width of mucosa); 4, moderate widespread; 5, moderate widespread and severe multifocal; 6, severe widespread. Lymphoid aggregates and gland abscesses (adenitis) were graded according to the actual numbers of glands affected. Parietal cell atrophy was graded as follows: mild, 1 to 10 glands; moderate, 10 to 20 glands; severe, >20 glands. The severity of the submucosal inflammation (i.e., inflammatory cells under the muscularis mucosa or in the nonglandular region) was graded as follows: 1, mild multifocal, few cells; 2, mild widespread, thin sheet of cells; 3, moderate multifocal, areas of moderate numbers of cells; 4, moderate widespread, moderate sheets of cells; 5, severe multifocal, areas of dense cells; 6, severe widespread, dense sheets of cells.
For β-galactosidase activity detection, paraffin sections (7 μm) were incubated in a 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) solution at 32°C for 16 h as previously described (1). The cells containing β-galactosidase-positive blue nuclei were counted blind (by P.A.). The mean numbers of these cells in stomach samples were calculated by dividing the total number of blue nuclei by the number of fields included in each section at a magnification of ×20. For immunohistochemical staining of macrophages, tissue sections were incubated with rat anti-mouse F4/80 antibody (1:75; Caltag, Tebu-Bio SA, Le Perray en Yvelines, France) for 2 h at 25°C. Endogenous peroxidase was inactivated (0.3% H2O2 in PBS). Tissue sections were then incubated with biotin-labeled polyclonal rabbit anti-rat immunoglobulin (1:400; Dako, Trappes, France), followed by incubation for 1 h with streptavidin-conjugated horseradish peroxidase (1:600; GE Healthcare, Les Ulis, France) and 10 min with aminoethylcarbazole (Sigma, Lyon, France). Detection of antigen-antibody complexes was performed with the DakoCytomation EnVision system (horseradish peroxidase-labeled anti-rabbit polymer; Dako, Trappes, France) according to the manufacturer's instructions. Tissue sections were counterstained with Harris' hematoxylin-eosin stain.
Gastric homogenates.
Gastric biopsy samples in PBS containing protease inhibitors were homogenized with an Ultra-Turrax homogenizer (IKA, Staufen, Germany). The samples were centrifuged at 12,000 × g for 10 min at 4°C, and the supernatants were collected for cytokine ELISA. Total protein concentrations of the supernatants were determined with the Bradford assay (Bio-Rad Laboratories, Hercules, CA).
Chemokine assays.
CXC chemokine (KC, MIP-2, or IL-8) levels were determined from culture and/or gastric mucosal supernatants with DuoSet cytokine ELISA kits according to the manufacturer's (R&D Systems) instructions.
Statistical analysis.
Data were analyzed with the Student t test (two sided), the Mann-Whitney test, or contingency tables, as appropriate. Differences between values were considered significant at P ≤ 0.05.
RESULTS
cagPAI-independent induction of CXC chemokine production in murine GECs.
The responses of murine cells to Helicobacter stimulation were studied with the GSM06 GEC line. These cells were cocultured with mouse-colonizing H. pylori strain 245 or SS1 or with H. felis. H. pylori strain 245 is a cagPAI-positive clinical isolate that was shown to colonize mice at low levels (31). Consistent with the presence of a functional cagPAI, this strain induced NF-κB reporter activity and IL-8 production in human GECs (31). In contrast, H. pylori SS1 is a mouse-adapted strain with a genetically intact but nonfunctional cagPAI (6, 31). The cagPAI-negative gastric Helicobacter species H. felis was included in these studies as it is known to induce more severe gastritis in mice than H. pylori (35).
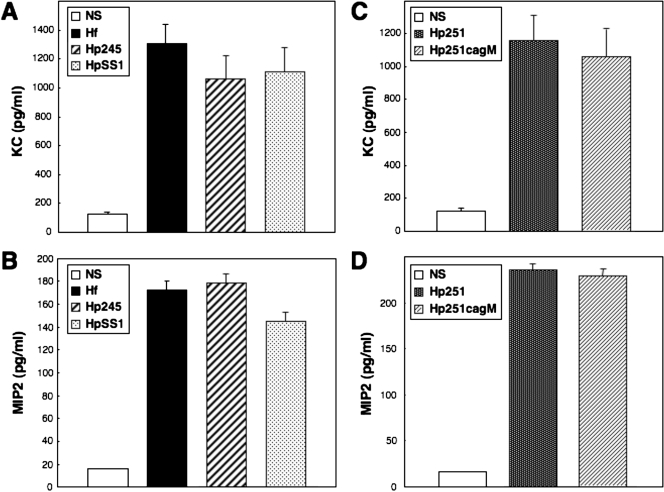
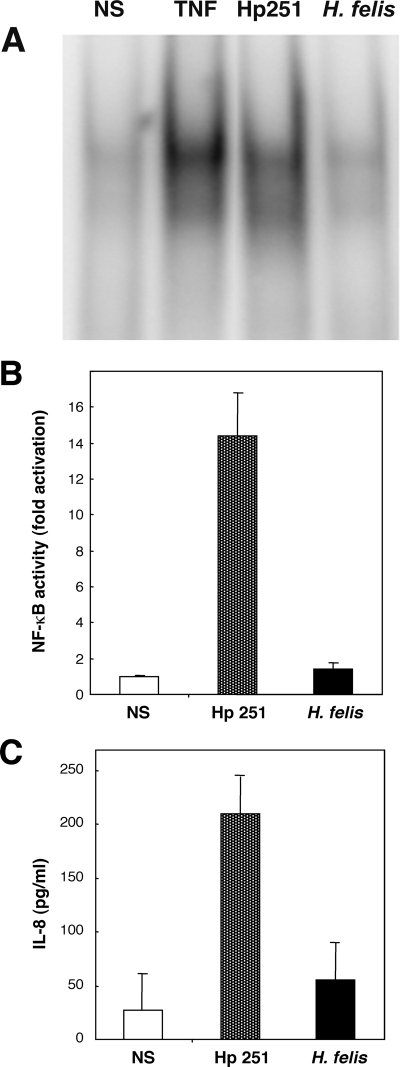
All of the H. pylori and H. felis strains tested were able to induce similar levels of KC and MIP-2 production in GSM06 cells (Fig. 1). As neither H. pylori SS1 (6, 31) nor H. felis (48) possesses a functional cagPAI, it seemed that this virulence factor is not required for induction of CXC chemokine synthesis in murine GECs. This was confirmed with a cagPAI-positive H. pylori clinical isolate (strain 251) and its isogenic cagM mutant. The lack of a functional cagPAI in the H. pylori 251 cagM mutant was previously confirmed by its inability to induce NF-κB reporter activity or IL-8 production in AGS cells (48). This strain was, however, still able to induce CXC chemokine production in GSM06 cells (Fig. 1), thus indicating the role of a cagPAI-independent mechanism in these responses. This contrasts with the situation in GEC lines of human origin (4, 21, 37). Also, we showed that H. felis, in common with cagPAI-negative H. pylori strains, did not induce NF-κB activation or IL-8 production in AGS cells, a human GEC line (Fig. 2).
FIG. 1.
H. pylori and H. felis strains increase KC and MIP-2 production in mouse GECs in a cagPAI-independent manner. KC (A and C) and MIP-2 (B and D) production in culture supernatants of nonstimulated (NS) cells and in cells cocultured for 18 to 24 h with H. felis (Hf), H. pylori 245 (Hp245), H. pylori SS1 (HpSS1), H. pylori 251 (Hp251), or an isogenic cagPAI mutant H. pylori 251 (Hp251cagM). Data correspond to the mean ± the standard error of the mean (triplicate determinations) and are representative of three to five independent experiments. Statistically significant differences in KC or MIP-2 levels, compared to those of control cells, were observed following bacterial stimulation (P < 0.0001). No significant differences were observed in KC or MIP-2 levels following bacterial stimulation with H. pylori 251 or 251 cagM mutant bacteria (P = 0.30 and P = 0.12, respectively).
FIG. 2.
H. felis is unable to induce NF-κB activation or IL-8 responses in AGS cells. NF-κB activation was determined by either EMSA (A) or a luciferase reporter assay (B) in nonstimulated (NS) cells and in those cocultured with H. pylori 251 (Hp 251) or H. felis. NF-κB binding and reporter activities were assessed at 2 and 6 h poststimulation, respectively. Human tumor necrosis factor (TNF) was used as a positive control in the EMSA. (C) The quantities of IL-8 in culture supernatants of nonstimulated (NS) cells and in those cocultured with H. pylori 251 (Hp 251) or H. felis were measured after 18 to 24 h of stimulation. Luciferase and IL-8 data correspond to the mean ± the standard error of the mean (triplicate determinations) and are representative of three independent experiments. Increased levels of NF-κB reporter activity and IL-8 production were observed for cells stimulated with H. pylori 251 (P < 0.05 and P < 0.001, respectively) but not in H. felis-stimulated cells (P > 0.05 versus control cells).
Inhibition of CXC chemokine production in murine GECs.
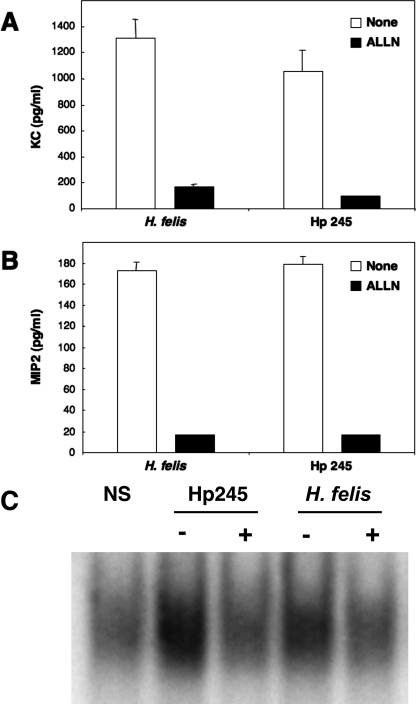
It has been reported that murine KC and MIP-2 gene expression is regulated by NF-κB (44, 50). To confirm the role of this transcription factor in Helicobacter-induced CXC chemokine production in GSM06 cells, we pretreated cells with the proteasome inhibitor ALLN. This inhibitor prevents the degradation of IκB family proteins by the 26S proteasome and thus interferes with the release of activated NF-κB complexes into the nucleus. ALLN pretreatment abrogated KC and MIP-2 synthesis by GSM06 cells in response to stimulation with H. pylori 245 or H. felis (Fig. 3A and B). Similar results were obtained with the proteasome inhibitors MG-132 and PDTC (data not shown). Moreover, ALLN pretreatment blocked NF-κB activation in EMSA (Fig. 3C), thus confirming the role of NF-κB in Helicobacter-induced KC and MIP-2 production in GSM06 cells.
FIG. 3.
Proteasome inhibition abrogates Helicobacter-induced KC and MIP-2 production in mouse GECs. KC (A) and MIP-2 (B) production was measured in culture supernatants of GSM06 cells that had been pretreated or not pretreated with the proteasome inhibitor ALLN and then stimulated for 24 to 26 h with either H. felis or H. pylori 245 (Hp 245). (C) NF-κB binding activity was determined by EMSA in GSMO6 cells treated (+) or not treated (−) with ALLN and then stimulated with bacteria. Chemokine data correspond to the mean ± the standard error of the mean (triplicate determinations) and are representative of three independent experiments. Statistically significant differences in chemokine production were observed between untreated and ALLN-pretreated cells (P < 0.0001).
Colonization of mice used to study NF-κB activation in vivo.
Given that NF-κB was involved in Helicobacter-induced CXC chemokine production in mouse GECs, we next sought to study the activation of this transcription factor during infection in vivo. For this, we used transgenic mice in which an NF-κB-responsive promoter element drives a lacZ reporter gene containing a nuclear localization sequence at its 5′ terminus (20, 36). These κB-lacZ mice were challenged with H. felis or H. pylori strain 245 or SS1. H. felis and H. pylori SS1 efficiently colonized the mice, with 10/11 animals in both groups culture positive for Helicobacter infection at day 1 postinoculation versus 4/11 positive in the group challenged with H. pylori 245 (P = 0.027; Table 1). At day 30, all of the mice challenged with either H. felis or H. pylori SS1 were positive by culture and/or serology versus 7/10 mice inoculated with H. pylori 245 (P > 0.05). Consistent with previous findings (31), mice challenged with H. pylori 245 exhibited bacterial loads significantly lower than those of H. pylori SS1-infected animals at days 1 and 30 postinoculation (P < 0.015 and P < 0.0001, respectively; Table 1).
TABLE 1.
Infection status and bacterial loads in κB-lacZ transgenic mice challenged with different Helicobacter isolates
| Mouse group | Infection time (days) | Helicobacter infection status (no. positive/total [n = 6-9 stomachs])a | Bacterial load (mean log no. of CFU/g ± SEM) |
|---|---|---|---|
| Naive | 1 | 0/11 | <3.0 |
| H. felis infected | 1 | 10/11 | NRb |
| H. pylori 245 infected | 1 | 4/11 | 3.74 ± 0.63c |
| H. pylori SS1 infected | 1 | 10/11 | 4.97 ± 0.81 |
| Naive | 30 | 0/11 | <3 |
| H. felis infected | 30 | 9/9 | NR |
| H. pylori 245 infected | 30 | 7/10 | 3.25 ± 0.39c |
| H. pylori SS1 infected | 30 | 9/9 | 6.24 ± 0.75 |
The results (from two or three independent experiments) were determined by culture alone or culture and serology, respectively, at days 1 and 30 postinoculation.
NR, not reported because H. felis bacteria isolated from gastric biopsies do not form well-isolated colonies on agar plates.
H. pylori 245 bacterial loads were significantly lower than those of H. pylori SS1 at days 1 and 30 postinoculation (P < 0.015 and P < 0.0001, respectively).
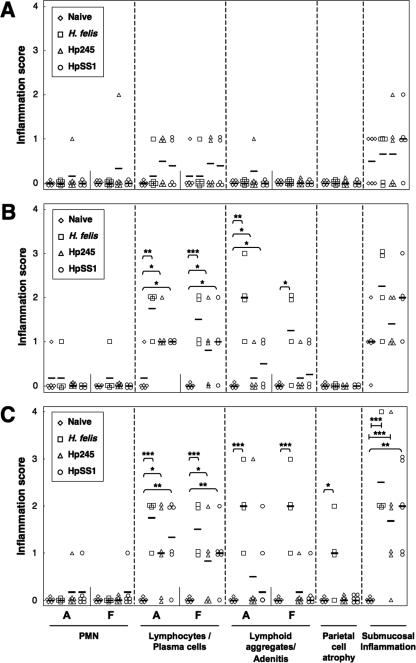
The gastric inflammatory changes in the stomachs of Helicobacter-infected κB-lacZ mice were characterized by lymphocytic and plasma cell infiltration of the lamina propria, as well as the formation of inflammatory cell aggregates and gland abscesses (adenitis) (Fig. 4). The severity of the changes in animals from all Helicobacter-infected groups increased over time and affected both the antral and fundic tissues. The observed changes were particularly pronounced in H. felis-infected mice, which also developed significant levels of parietal cell atrophy at day 135 postinoculation (Fig. 4C). Submucosal inflammation, as characterized by the presence of inflammatory cells under the muscularis mucosa or in the nonglandular region of the tissues, was observed in all groups of Helicobacter-infected mice. Nevertheless, these lesions were only significantly more severe in mice with long-term Helicobacter infection (135 days) compared to naive animals (Fig. 4C).
FIG. 4.
Inflammatory lesions in the gastric mucosa of κB-lacZ mice in response to Helicobacter infection. Mice were either administered broth alone (Naive) or challenged with bacterial suspensions of H. felis, H. pylori 245 (Hp245), or H. pylori SS1 (HpSS1). Animals were sacrificed at day 1 (A), 30 (B), or 135 (C) postchallenge. Tissue sections from the mice were assessed for PMN and lymphocyte/plasma cell infiltration in the lamina propria of the antrum (A) and fundus (F), lymphoid aggregates and gland abscesses (adenitis), parietal cell atrophy, and submucosal inflammation. Each datum point corresponds to the scores of an individual animal, whereas the horizontal bars represent the mean score per group (four to six animals per group). Statistically significant differences between Helicobacter-infected and naive animals are shown as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.005.
Helicobacter-induced NF-κB activation and CXC chemokine production in vivo.
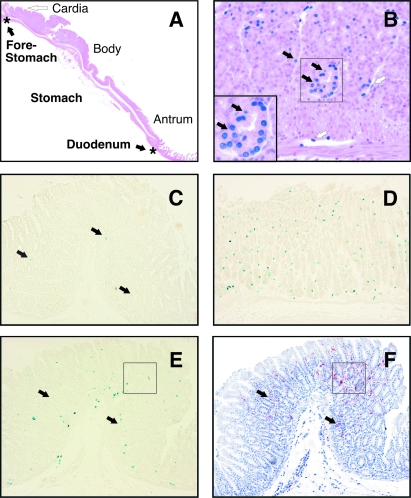
The levels of NF-κB activation in the gastric tissues of Helicobacter-challenged and naive mice were quantified by assessing β-galactosidase staining in cell nuclei, which is indicative of specific NF-κB activation. These analyses were performed on all regions of the stomach, from the cardia to the antrum (Fig. 5A). β-Galactosidase activity was observed primarily in epithelial cells but also in other cell types, including endothelial cells, scattered throughout the mucosa and submucosa (Fig. 5B). Cells with β-galactosidase activity were distributed unevenly throughout the gastric tissues of both naive and infected mice, with some tissue areas containing very low numbers of β-galactosidase-positive cells (Fig. 5C), whereas other areas contained higher densities of these cells (Fig. 5D and E). A similar patchy distribution of β-galactosidase-positive cells was also observed within the gastric tissues of the same animal (data not shown). No obvious areas of colocalization could be observed between β-galactosidase-positive cells and Mac-1+ (CD11b) inflammatory cells, such as macrophages and dendritic cells, in Helicobacter-infected mice with inflammatory lesions (Fig. 5E and F). Thus, it appears that NF-κB activation can occur distant from the site of inflammation.
FIG. 5.
Histological detection of NF-κB activation in H. pylori-infected mice. (A) Gastric tissue segments were collected from mice either administered broth alone or challenged with Helicobacter strains. Tissue segments extended from the proximal portion of the mouse stomach, the forestomach, to the distal region adjacent to the duodenum and included the major gastric regions of the cardia, body, and antrum. (B to D) Detection of NF-κB-responsive β-galactosidase activity within tissue sections from naive (C) and Helicobacter-infected (B and D) mice. Nuclear β-galactosidase activity was detected in epithelial (B and C, black arrows) and endothelial (B, white arrows) cells (X-Gal and hematoxylin-eosin staining). (B, insert) Enlarged view of a gastric gland (indicated by a box) within the body region showing numerous epithelial cells (black arrows) with nuclear β-galactosidase activity. The tissue was taken from an H. felis-infected mouse at day 30 postinfection. (C and D) β-Galactosidase-positive cells in noncounterstained tissues from naive (C) and H. pylori 245-infected (D) mice at days 30 and 1 postinoculation, respectively. Absence of colocalization of β-galactosidase-positive (E) and Mac1+ (F) inflammatory cells in sequential tissue sections taken from a mouse stomach at 30 days postinfection with H. felis. Tissue foci containing Mac1+ cells in panels E and F are indicated by black arrows and a box. The section in panel E was counterstained with the Giemsa stain. Magnifications: A, ×4; B, ×40; C, E, and F, ×10; D, ×20.
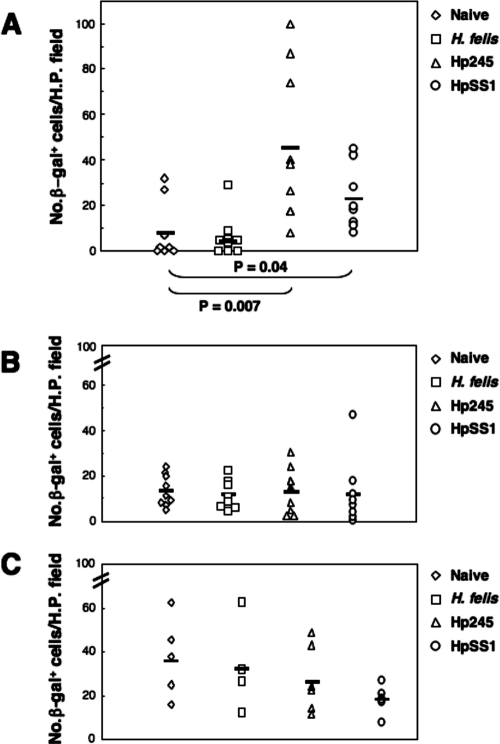
In order to quantify the level of NF-κB activation in the mice, the numbers of β-galactosidase-positive cells per high-power field in all tissue sections from a single stomach were determined and the mean numbers of cells were determined. Significant increases in the mean numbers of β-galactosidase-positive cells per high-power field were observed in the gastric mucosa of mice at day 1 postchallenge with H. pylori strains 245 and SS1 (P = 0.007 and P = 0.04, respectively, versus naive animals) but not in those receiving H. felis (Fig. 6A). At days 30 and 135 postchallenge, β-galactosidase-positive cells were detected in the gastric tissue sections of nearly all mice, including those of naive animals (Fig. 6B and C). At these time points, however, the mean numbers of β-galactosidase-positive cells of infected mice did not differ significantly from those in naive animals. Preliminary experiments with small numbers of animal (two per group) at 1 week postinoculation also found no differences between the different groups (data not shown). These data demonstrate that NF-κB is rapidly activated in response to gastric mucosal infection with H. pylori bacteria.
FIG. 6.
Rapid induction of NF-κB activation in H. pylori-infected mice. Mice were either administered broth alone (Naive) or challenged with bacterial suspensions of H. felis, H. pylori 245 (Hp245), or H. pylori SS1 (HpSS1). Animals were sacrificed at 1 (A), 30 (B), or 135 (C) days postchallenge. The levels of NF-κB activation was determined by histological assessment of gastric biopsy samples for the presence of NF-κB-responsive β-galactosidase (β-gal) activity in cell nuclei. Symbols correspond to the mean numbers of cells with β-galactosidase-positive nuclei counted per high-power (H.P.) field from tissue sections taken from a single mouse. The horizontal bars correspond to the mean numbers of β-galactosidase-positive cells counted for each group. The data for the day 1 and 30 time points were pooled from three independent experiments. Statistically significant differences were observed at day 1 postinoculation between mice infected with H. pylori strains 245 and SS1, compared to naive animals (P = 0.007 and P = 0.04, respectively).
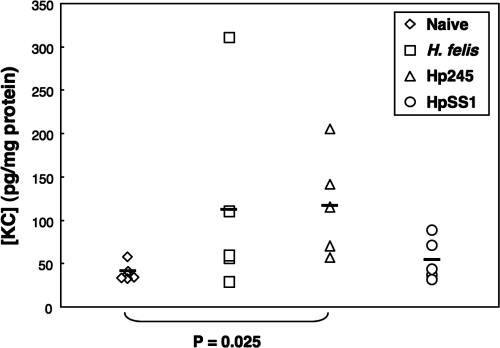
To determine whether NF-κB activation could be correlated with increased mucosal levels of CXC chemokines, we measured the presence of KC and MIP-2 in gastric tissue homogenates at day 1 postinoculation. The levels of KC were significantly increased in the gastric homogenates of H. pylori 245-infected mice, compared to naive controls (P = 0.025), whereas no significant differences were observed for tissues from H. pylori SS1- and H. felis-infected animals (Fig. 7). The levels of MIP-2 were undetectable in all of the samples tested (data not shown).
FIG. 7.
Increased levels of mucosal KC in H. pylori 245-infected mice. Levels of KC were measured in tissue homogenates taken from naive mice, as well as animals at day 1 postinoculation with H. felis, H. pylori 245 (Hp245), or H. pylori SS1 (HpSS1). Statistically significantly increased levels were only observed in mice infected with H. pylori 245, compared to naive animals (P = 0.025).
DISCUSSION
Mouse models have been extensively used to study the inflammatory responses induced by H. pylori infection. This animal host, however, lacks IL-8, which is known to be a key mediator of the inflammatory responses in H. pylori-infected individuals. In the present study, we have shown that KC and MIP-2, two murine CXC chemokines that have been reported to have biological functions analogous to those of human IL-8 (29, 32), are produced by the murine GSM06 GEC line in response to stimulation with H. pylori strains and H. felis (Fig. 1). Moreover, we have shown for the first time with both wild-type and isogenic mutant bacteria that the cagPAI is not required for CXC chemokine responses in murine GECs (Fig. 1), suggesting host-specific differences in H. pylori-GEC interactions. Nevertheless, in common with proinflammatory responses to H. pylori in human GECs (21, 37), those in murine cells were dependent upon activation of the transcription factor NF-κB (Fig. 2). This finding concurred with studies with κB-lacZ transgenic mice in which mouse-colonizing cagPAI-positive and -negative H. pylori strains induced significantly higher numbers of β-galactosidase-positive cells, indicative of NF-κB activation, compared to naive mice (Fig. 6). Strikingly, these responses were observed only in the acute, and not in the chronic, phase of H. pylori infection and not in H. felis-infected animals. This is the first evidence suggesting that helicobacters may be able to modulate the levels of activated NF-κB complexes during infection of the mouse gastric mucosa.
Previous reports described up-regulated gene expression for various proinflammatory mediators in Helicobacter-stimulated GSM06 cells (8, 27). The present work extends these findings by showing, for the first time, the synthesis of two such mediators, KC and MIP-2, by GSM06 cells in response to bacterial stimulation (Fig. 1). Moreover, we have now shown (Fig. 1) that H. pylori induces NF-κB activation in GECs of murine origin via a primarily cagPAI-independent mechanism, thus differing from the situation in human-derived or Mongolian-gerbil-derived cells (21, 26, 37).
Studies in the κB-lacZ mouse model (Fig. 4 to 6) suggest that a functional cagPAI is also dispensable for proinflammatory responses in vivo. This conclusion could not, however, be confirmed in the present work because the H. pylori 245 strain is not naturally transformable and therefore cannot be manipulated genetically (R.L.F., data not shown). Nevertheless, Yamaoka et al. (51) reported only a slight reduction in gastric inflammation and KC synthesis in mice infected with an H. pylori isogenic cagPAI mutant, compared to animals infected with the wild-type strain. In contrast, decreased levels of inflammation were observed in Mongolian gerbils infected with H. pylori cagPAI mutants (28, 33, 38). The differences in the responsiveness of these rodent species to cagPAI-positive H. pylori strains cannot be explained solely by the absence of IL-8 in mice because gerbils also do not produce this chemokine. From the in vitro and in vivo data, it appears that host cell-specific factors (2) may account for the differences in H. pylori-induced inflammation that have been observed in the various animal hosts. Given the contrasting responses of mouse versus human GECs to H. felis stimulation (Fig. 1 and 2), it is likely that gastric Helicobacter spp. use different strategies to induce proinflammatory responses in animal hosts.
The mechanisms and signaling pathways involved in H. pylori induction of NF-κB responses in human GECs have been studied in great detail in vitro, yet the in vivo relevance of these observations has only rarely been investigated. Activated NF-κB complexes were detected by immunohistochemistry within the gastric mucosa of individuals chronically infected with H. pylori (17, 18, 46). Consistent with the work presented here (Fig. 5), most of the activated NF-κB complexes in those studies were associated with the nuclei of epithelial cells (17, 18, 46). Although one report specifically referred to NF-κB activation in antral GECs, only antral samples were analyzed in that study (46). As found here, other workers reported NF-κB activation in GECs throughout the body and antral tissues of H. pylori-infected individuals (17, 18).
A correlation was previously reported in H. pylori-infected subjects for gastric mucosal levels of NF-κB activation and PMN infiltration (17, 18). No such correlation could, however, be found in Helicobacter-infected κB-lacZ mice at any time point. Although the reasons for this finding are unclear, significantly increased mucosal levels of KC, a key chemokine for PMN recruitment and activation in mice, were observed in mice infected with H. pylori 245 at day 1 postinoculation (Fig. 7). H. pylori 245-infected mice also showed the greatest numbers of β-galactosidase-positive cells in their gastric mucosa (Fig. 6A). These findings are consistent with the reported association between NF-κB activation and raised mucosal levels of the analogous CXC chemokine IL-8 in clinical samples (17, 18, 46). The inflammatory lesions in human hosts with H. pylori infection, however, differ from those in Helicobacter-infected mice. Indeed, there have been contradictory reports regarding the severity of PMN recruitment to the stomachs of Helicobacter-infected mice (7, 22, 23, 35), suggesting that this cell population may be a relatively minor component of the inflammatory lesions present in mouse Helicobacter infection models. Thus, to observe a correlation between NF-κB activation and PMN infiltration in Helicobacter-infected mice, it may be necessary to use more sensitive techniques than classical histopathology and/or to quantify the actual numbers of PMNs within the gastric mucosa. Alternatively, it is possible that the local synthesis of other proinflammatory factors may correlate more directly with NF-κB activation during acute H. pylori infection in mice.
The most curious and unexpected finding from the present work was the absence of a significant induction of NF-κB activation in H. felis-infected κB-lacZ mice. H. felis was originally included in this study because it induces a more severe gastritis than does H. pylori in mice (35) and was therefore expected to be a more a potent inducer of NF-κB activation in vivo. It seems unlikely that host factors are responsible for these findings as the κB-lacZ mice were maintained on a mixed background of two H. felis-responsive mouse strains (7, 35). Indeed, significant histopathology was detected in the gastric mucosa of the mice in spite of the lack of increased levels of NF-κB activation in epithelial cells (Fig. 4 and 5F). Another explanation is that H. felis, or indeed H. pylori strains, may be able to dampen proinflammatory responses in these cells. As reported for other Helicobacter spp. (39, 49), this may occur through direct effects on GECs or indirectly via the local production of antiinflammatory cytokines or induction of immunosuppressive cell populations (40). The capacity of H. pylori strains to down-regulate the host immune response in mice (10, 31, 47) clearly merits further investigation.
Acknowledgments
This work was funded by a Projet Transversal de Recherche (PTR38) from the Institut Pasteur to R.L.F., P.A., D.J.P., and S.M. R.L.F. is supported by project grants from the National Health and Medical Research Council of Australia. D.J.P. is funded by The Canadian Institutes of Health and is an International Research Scholar of the Howard Hughes Medical Institute.
S.M. is from the INSERM and is supported by grants from the CNRS and the Institut Pasteur.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 10 December 2007.
REFERENCES
- 1.Avé, P., E. Colucci-Guyon, C. Babinet, and M. R. Huerre. 1997. An improved method to detect β-galactosidase activity in transgenic mice: a post-staining procedure on paraffin embedded tissue sections. Transgenic Res. 637-40. [DOI] [PubMed] [Google Scholar]
- 2.Bauer, B., S. Moese, S. Bartfeld, T. F. Meyer, and M. Selbach. 2005. Analysis of cell type-specific responses mediated by the type IV secretion system of Helicobacter pylori. Infect. Immun. 734643-4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandt, S., T. Kwok, R. Hartig, W. Konig, and S. Backert. 2005. NF-κB activation and potentiation of proinflammatory responses by the Helicobacter pylori CagA protein. Proc. Natl. Acad. Sci. USA 1029300-9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Censini, S., C. Lange, Z. Xiang, J. E. Crabtree, P. Ghiara, M. Borodovsky, R. Rappuoli, and A. Covacci. 1996. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc. Natl. Acad. Sci. USA 9314648-14653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crabtree, J. E., A. Covacci, S. M. Farmery, Z. Xiang, D. S. Tompkins, S. Perry, I. J. D. Lindley, and R. Rappuoli. 1995. Helicobacter pylori induced interleukin-8 expression in gastric epithelial cells is associated with CagA positive phenotype. J. Clin. Pathol. 4841-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crabtree, J. E., R. L. Ferrero, and J. G. Kusters. 2002. The mouse colonizing Helicobacter pylori strain SS1 may lack a functional cag pathogenicity island. Helicobacter 7139-141. [DOI] [PubMed] [Google Scholar]
- 7.De Bock, M., A. Decostere, K. Van den Bulck, M. Baele, L. Duchateau, F. Haesebrouck, and R. Ducatelle. 2005. The inflammatory response in the mouse stomach to Helicobacter bizzozeronii, Helicobacter salomonis and two Helicobacter felis strains. J. Comp. Pathol. 13383-91. [DOI] [PubMed] [Google Scholar]
- 8.Debreceni, A., K. Okazaki, Y. Matsushima, M. Ohana, H. Nakase, K. Uchida, S. Uose, and T. Chiba. 2001. mRNA expression of cytokines and chemokines in the normal gastric surface mucous epithelial cell line GSM06 during bacterial infection with Helicobacter felis. J. Physiol. Paris 95461-467. [DOI] [PubMed] [Google Scholar]
- 9.Eaton, K. A., S. J. Danon, S. Krakowka, and S. E. Weisbrode. 2007. A reproducible scoring system for quantification of histologic lesions of inflammatory disease in mouse gastric epithelium. Comp. Med. 5757-65. [PubMed] [Google Scholar]
- 10.Eaton, K. A., D. Kersulyte, M. Mefford, S. J. Danon, S. Krakowka, and D. E. Berg. 2001. Role of Helicobacter pylori cag region genes in colonization and gastritis in two animal models. Infect. Immun. 692902-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrero, R. L., P. Avé, F. J. Radcliff, A. Labigne, and M. R. Huerre. 2000. Outbred mice with long-term Helicobacter felis infection develop both gastric lymphoid tissue and glandular hyperplastic lesions. J. Pathol. 191333-340. [DOI] [PubMed] [Google Scholar]
- 12.Ferrero, R. L., J. M. Thiberge, M. Huerre, and A. Labigne. 1998. Immune responses of specific-pathogen-free mice to chronic Helicobacter pylori (strain SS1) infection. Infect. Immun. 661349-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrero, R. L., J. M. Thiberge, M. Huerre, and A. Labigne. 1994. Recombinant antigens prepared from the urease subunits of Helicobacter spp.: evidence of protection in a mouse model of gastric infection. Infect. Immun. 624981-4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garhart, C. A., F. P. Heinzel, S. J. Czinn, and J. G. Nedrud. 2003. Vaccine-induced reduction of Helicobacter pylori colonization in mice is interleukin-12 dependent but gamma interferon and inducible nitric oxide synthase independent. Infect. Immun. 71910-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayden, M. S., A. P. West, and S. Ghosh. 2006. NF-κB and the immune response. Oncogene 256758-6780. [DOI] [PubMed] [Google Scholar]
- 16.Hirata, Y., S. Maeda, T. Ohmae, W. Shibata, A. Yanai, K. Ogura, H. Yoshida, T. Kawabe, and M. Omata. 2006. Helicobacter pylori induces IκB kinase α nuclear translocation and chemokine production in gastric epithelial cells. Infect. Immun. 741452-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isomoto, H., M. Miyazaki, Y. Mizuta, F. Takeshima, K. Murase, K. Inoue, K. Yamasaki, I. Murata, T. Koji, and S. Kohno. 2000. Expression of nuclear factor-κB in Helicobacter pylori-infected gastric mucosa detected with southwestern histochemistry. Scand. J. Gastroenterol. 35247-254. [DOI] [PubMed] [Google Scholar]
- 18.Isomoto, H., Y. Mizuta, M. Miyazaki, F. Takeshima, K. Omagari, K. Murase, T. Nishiyama, K. Inoue, I. Murata, and S. Kohno. 2000. Implication of NF-κB in Helicobacter pylori-associated gastritis. Am. J. Gastroenterol. 952768-2776. [DOI] [PubMed] [Google Scholar]
- 19.Jenks, P. J., C. Chevalier, C. Ecobichon, and A. Labigne. 2001. Identification of nonessential Helicobacter pylori genes using random mutagenesis and loop amplification. Res. Microbiol. 152725-734. [DOI] [PubMed] [Google Scholar]
- 20.Kayal, S., A. Lilienbaum, C. Poyart, S. Mémet, A. Israël, and P. Berche. 1999. Listeriolysin O-dependent activation of endothelial cells during infection with Listeria monocytogenes: activation of NF-κB and upregulation of adhesion molecules and chemokines. Mol. Microbiol. 311709-1722. [DOI] [PubMed] [Google Scholar]
- 21.Keates, S., Y. S. Hitti, M. Upton, and C. P. Kelly. 1997. Helicobacter pylori infection activates NF-κB in gastric epithelial cells. Gastroenterology 1131099-1109. [DOI] [PubMed] [Google Scholar]
- 22.Lee, A., J. O'Rourke, M. C. D. Ungria, B. Robertson, G. Daskalopoulos, and M. F. Dixon. 1997. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology 1121386-1397. [DOI] [PubMed] [Google Scholar]
- 23.Mohammadi, M., R. Redline, J. Nedrud, and S. Czinn. 1996. Role of the host in pathogenesis of Helicobacter-associated gastritis: H. felis infection of inbred and congenic mouse strains. Infect. Immun. 64238-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mori, N., A. Ueda, R. Geleziunas, A. Wada, T. Hirayama, T. Yoshimura, and N. Yamamoto. 2001. Induction of monocyte chemoattractant protein 1 by Helicobacter pylori involves NF-κB. Infect. Immun. 691280-1286. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Mori, N., A. Wada, T. Hirayama, T. P. Parks, C. Stratowa, and N. Yamamoto. 2000. Activation of intercellular adhesion molecule 1 expression by Helicobacter pylori is regulated by NF-κB in gastric epithelial cancer cells. Infect. Immun. 681806-1814. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Nozaki, K., H. Tanaka, Y. Ikehara, X. Cao, H. Nakanishi, T. Azuma, S. Yamazaki, Y. Yamaoka, N. Shimizu, K. Mafune, M. Kaminishi, and M. Tatematsu. 2005. Helicobacter pylori-dependent NF-kappa B activation in newly established Mongolian gerbil gastric cancer cell lines. Cancer Sci. 96170-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Obonyo, M., D. G. Guiney, J. Harwood, J. Fierer, and S. P. Cole. 2002. Role of gamma interferon in Helicobacter pylori induction of inflammatory mediators during murine infection. Infect. Immun. 703295-3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogura, K., S. Maeda, M. Nakao, T. Watanabe, M. Tada, T. Kyutoku, H. Yoshida, Y. Shiratori, and M. Omata. 2000. Virulence factors of Helicobacter pylori responsible for gastric diseases in Mongolian gerbil. J. Exp. Med. 1921601-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohtsuka, Y., J. Lee, D. S. Stamm, and I. R. Sanderson. 2001. MIP-2 secreted by epithelial cells increases neutrophil and lymphocyte recruitment in the mouse intestine. Gut 49526-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peek, R. M., Jr., G. G. Miller, K. T. Tham, G. I. Pérez-Pérez, X. Zhao, J. C. Atherton, and M. J. Blaser. 1995. Heightened inflammatory response and cytokine expression in vivo to cagA+ Helicobacter pylori strains. Lab. Investig. 73760-770. [PubMed] [Google Scholar]
- 31.Philpott, D. J., D. Belaid, P. Troubadour, J.-M. Thiberge, J. Tankovic, A. Labigne, and R. L. Ferrero. 2002. Reduced activation of inflammatory responses in host cells by mouse-adapted Helicobacter pylori isolates. Cell. Microbiol. 4285-296. [DOI] [PubMed] [Google Scholar]
- 32.Remick, D. G., L. B. Green, D. E. Newcomb, S. J. Garg, G. L. Bolgos, and D. R. Call. 2001. CXC chemokine redundancy ensures local neutrophil recruitment during acute inflammation. Am. J. Pathol. 1591149-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rieder, G., J. L. Merchant, and R. Haas. 2005. Helicobacter pylori cag-type IV secretion system facilitates corpus colonization to induce precancerous conditions in Mongolian gerbils. Gastroenterology 1281229-1242. [DOI] [PubMed] [Google Scholar]
- 34.Saito, H., Y. Yamaoka, S. Ishizone, F. Maruta, A. Sugiyama, D. Y. Graham, K. Yamauchi, H. Ota, and S. Miyagawa. 2005. Roles of virD4 and cagG genes in the cag pathogenicity island of Helicobacter pylori using a Mongolian gerbil model. Gut 54584-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakagami, T., M. Dixon, J. O'Rourke, R. Howlett, F. Alderuccio, J. Vella, T. Shimoyama, and A. Lee. 1996. Atrophic gastric changes in both Helicobacter felis and Helicobacter pylori infected mice are host dependent and separate from antral gastritis. Gut 39639-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt-Ullrich, R., S. Mémet, A. Lilienbaum, J. Feuillard, M. Raphaël, and A. Israël. 1996. NF-κB activity in transgenic mice: developmental regulation and tissue specificity. Development 1222117-2128. [DOI] [PubMed] [Google Scholar]
- 37.Sharma, S. A., M. K. R. Tummuru, G. G. Miller, and M. J. Blaser. 1995. Interleukin-8 response of gastric epithelial cell lines to Helicobacter pylori stimulation in vitro. Infect. Immun. 631681-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shibata, W., Y. Hirata, S. Maeda, K. Ogura, T. Ohmae, A. Yanai, Y. Mitsuno, Y. Yamaji, M. Okamoto, H. Yoshida, T. Kawabe, and M. Omata. 2006. CagA protein secreted by the intact type IV secretion system leads to gastric epithelial inflammation in the Mongolian gerbil model. J. Pathol. 210306-314. [DOI] [PubMed] [Google Scholar]
- 39.Sterzenbach, T., S. K. Lee, B. Brenneke, F. von Goetz, D. B. Schauer, J. G. Fox, S. Suerbaum, and C. Josenhans. 2007. Inhibitory effect of enterohepatic Helicobacter hepaticus on innate immune responses of mouse intestinal epithelial cells. Infect. Immun. 752717-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strömberg, E., A. Edebo, B. S. Lundin, P. Bergin, M. Brisslert, A. M. Svennerholm, and C. Lindholm. 2005. Down-regulation of epithelial IL-8 responses in Helicobacter pylori-infected duodenal ulcer patients depends on host factors, rather than bacterial factors. Clin. Exp. Immunol. 140117-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sugiyama, N., Y. Tabuchi, T. Horiuchi, M. Obinata, and M. Furusawa. 1993. Establishment of gastric surface mucous cell lines from transgenic mice harboring temperature-sensitive simian virus 40 large T-antigen gene. Exp. Cell Res. 209382-387. [DOI] [PubMed] [Google Scholar]
- 42.Tabuchi, Y., N. Sugiyama, T. Horiuchi, K. Furuhama, and M. Furusawa. 1996. Biological characterization of gastric surface mucous cell line GSM06 from transgenic mice harboring temperature-sensitive simian virus 40 large T-antigen gene. Digestion 57141-148. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi, T., T. Matsumoto, M. Nakamura, H. Matsui, H. Kiyohara, C. Sasakawa, and H. Yamada. 2004. A novel in vitro infection model of Helicobacter pylori using mucin-producing murine gastric surface mucous cells. Helicobacter 9302-312. [DOI] [PubMed] [Google Scholar]
- 44.Tebo, J. M., S. Datta, R. Kishore, M. Kolosov, J. A. Major, Y. Ohmori, and T. A. Hamilton. 2000. Interleukin-1-mediated stabilization of mouse KC mRNA depends on sequences in both 5′- and 3′-untranslated regions. J. Biol. Chem. 27512987-12993. [DOI] [PubMed] [Google Scholar]
- 45.Tomb, J.-F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, E. K. Hickey, D. E. Berg, J. D. Gocayne, T. R. Utterback, J. D. Peterson, J. M. Kelley, M. D. Cotton, J. M. Weidman, C. Fujii, C. Bowman, L. Watthey, E. Wallin, W. S. Hayes, M. Borodovsky, P. D. Karp, H. O. Smith, C. M. Fraser, and J. C. Venter. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388539-547. [DOI] [PubMed] [Google Scholar]
- 46.van den Brink, G. R., F. J. ten Kate, C. Y. Ponsioen, M. M. Rive, G. N. Tytgat, S. J. van Deventer, and M. P. Peppelenbosch. 2000. Expression and activation of NF-κB in the antrum of the human stomach. J. Immunol. 1643353-3359. [DOI] [PubMed] [Google Scholar]
- 47.van Doorn, N. E. M., F. Namavar, M. Sparrius, J. Stoof, E. P. van Rees, L.-J. van Doorn, and C. M. J. E. Vandenbroucke-Grauls. 1999. Helicobacter pylori-associated gastritis in mice is host and strain specific. Infect. Immun. 673040-3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Viala, J., C. Chaput, I. G. Boneca, A. Cardona, S. E. Girardin, A. P. Moran, R. Athman, S. Mémet, M. R. Huerre, A. J. Coyle, P. S. DiStefano, P. J. Sansonetti, A. Labigne, J. Bertin, D. J. Philpott, and R. L. Ferrero. 2004. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat. Immunol. 51166-1174. [DOI] [PubMed] [Google Scholar]
- 49.Walduck, A., A. Schmitt, B. Lucas, T. Aebischer, and T. F. Meyer. 2004. Transcription profiling analysis of the mechanisms of vaccine-induced protection against H. pylori. FASEB J. 181955-1957. [DOI] [PubMed] [Google Scholar]
- 50.Widmer, U., K. R. Manogue, A. Cerami, and B. Sherry. 1993. Genomic cloning and promoter analysis of macrophage inflammatory protein (MIP)-2, MIP-1 alpha, and MIP-1 beta, members of the chemokine superfamily of proinflammatory cytokines. J. Immunol. 1504996-5012. [PubMed] [Google Scholar]
- 51.Yamaoka, Y., M. Kita, T. Kodama, S. Imamura, T. Ohno, N. Sawai, A. Ishimaru, J. Imanishi, and D. Y. Graham. 2002. Helicobacter pylori infection in mice: role of outer membrane proteins in colonization and inflammation. Gastroenterology 1231992-2004. [DOI] [PubMed] [Google Scholar]
- 52.Yamaoka, Y., T. Kita, T. Kodama, N. Sawai, and J. Imanishi. 1996. Helicobacter pylori cagA gene and expression of cytokine messenger RNA in gastric mucosa. Gastroenterology 1101744-1752. [DOI] [PubMed] [Google Scholar]