Abstract
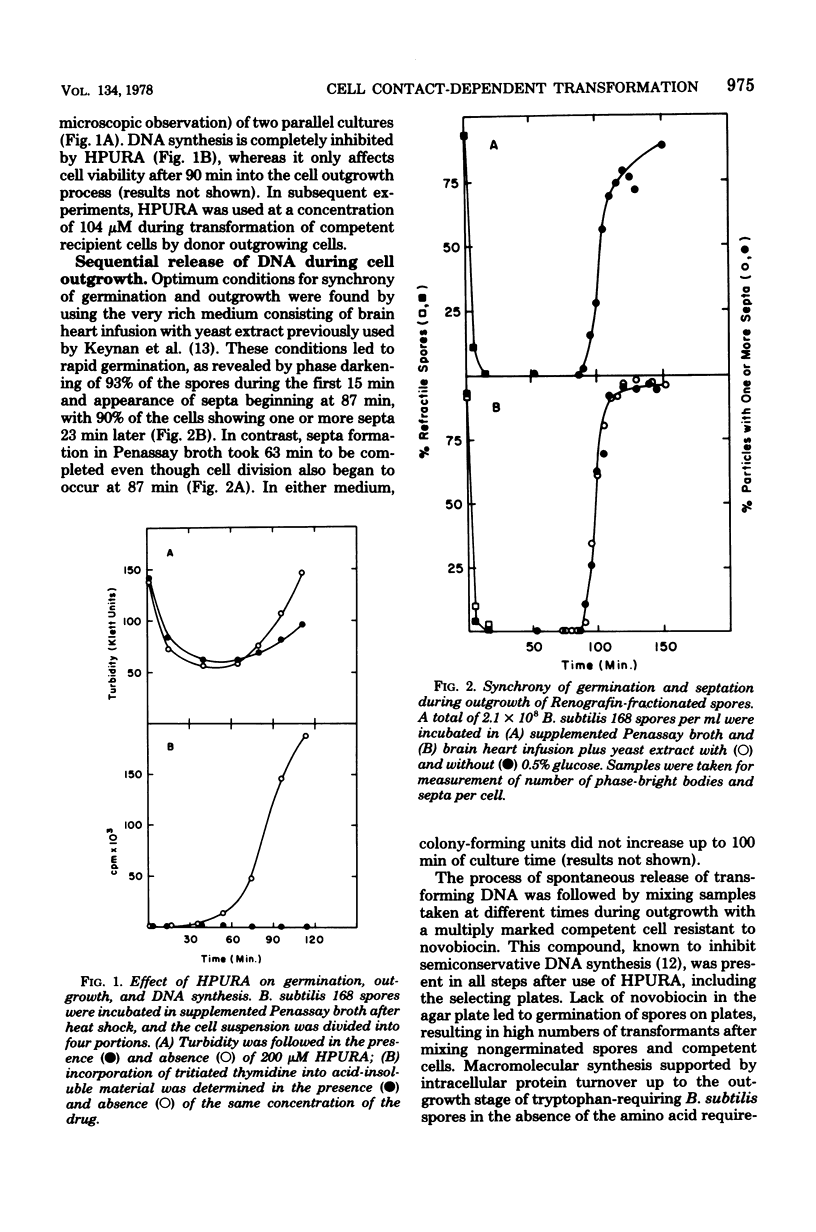
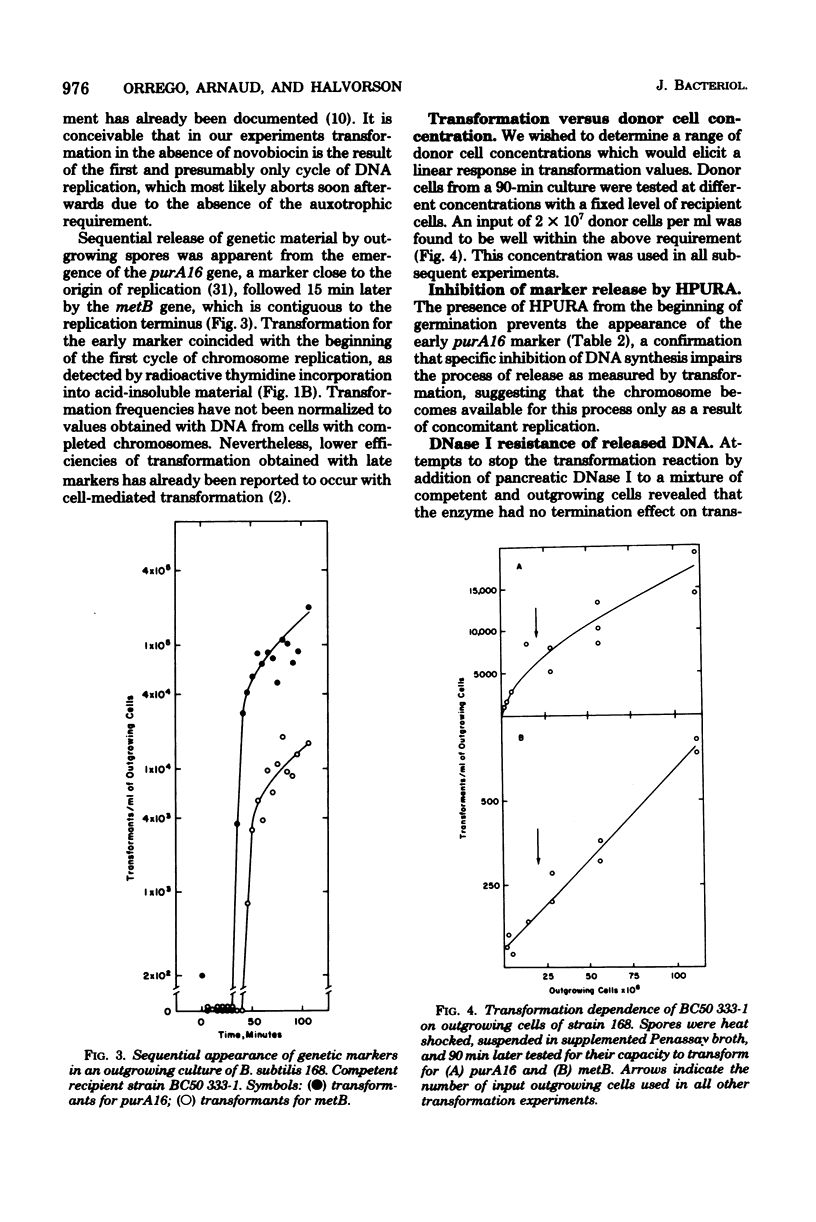
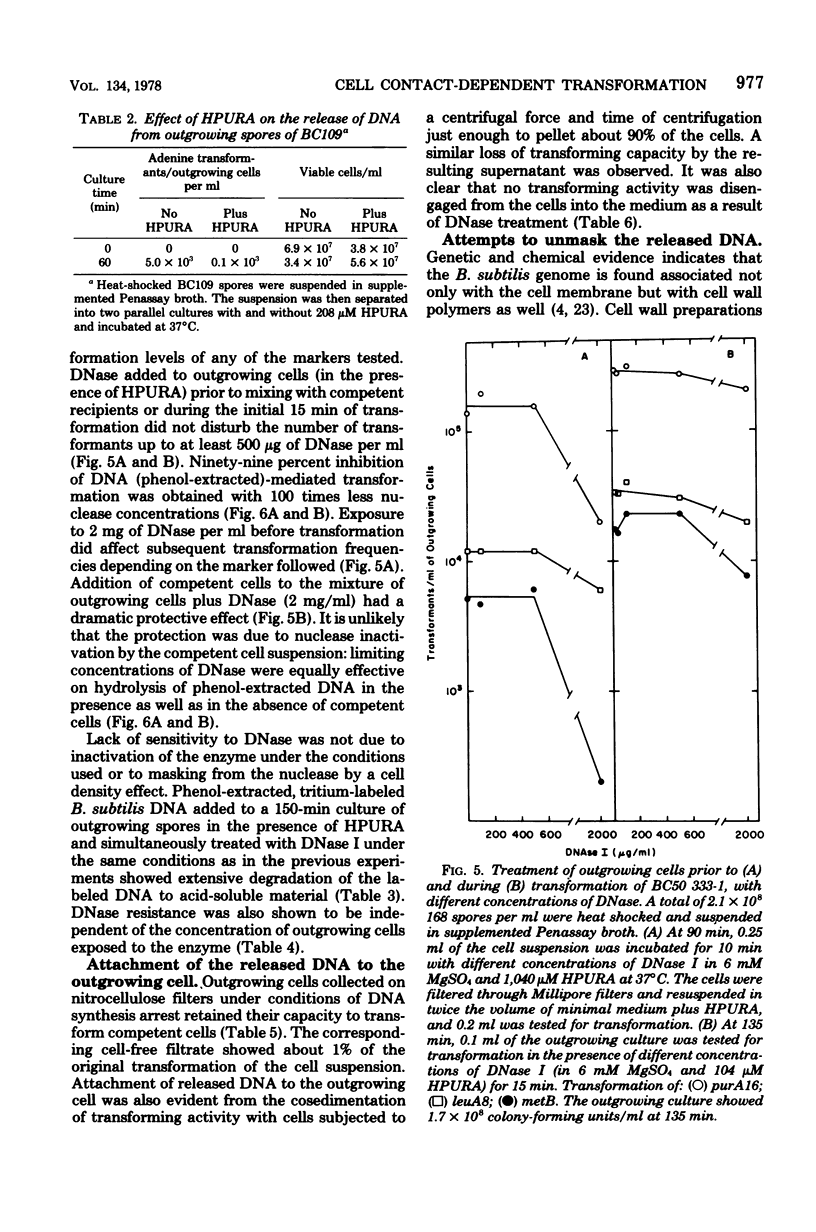
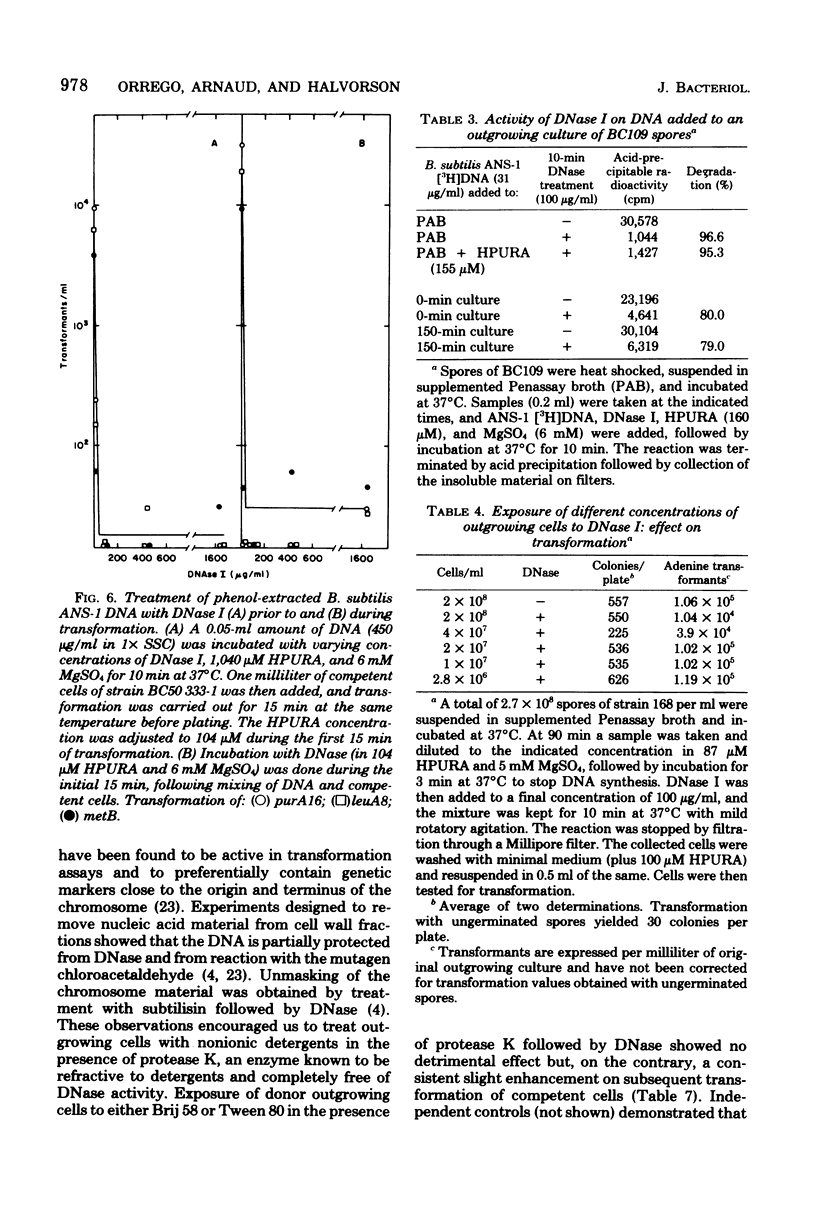
Transforming activity released in sequential genetic order during the first synchronous cycle of DNA replication during outgrowth of spores of Bacillus subtilis 168 was investigated. A transformation assay was used consisting of outgrowing spores as DNA donors and multiply marked competent cells as recipients. DNA synthesis inhibitors known to stop DNA release were used during and subsequent to DNA transfer to recipient cells. The released DNA sedimented with the outgrowing cells after low-speed centrifugation, and it was discovered that markers released both early and late were resistant to up to 500 microgram of deoxyribonuclease per ml under conditions in which the transforming capacity of purified DNA was eliminated by 5 microgram of the nuclease per ml. Inaccessibility to deoxyribonuclease was increased and maintained during the transformation event while detergents and proteolytic attack did not expose the released chromosome to nuclease action. The results indicate that tight physical contact between outgrowing spores and competent cells is required for transformation in this system.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein S., Ephrati-Elizur E. Spontaneous release of DNA in sequential genetic order by Bacillus subtilis. J Mol Biol. 1969 Oct 14;45(1):137–152. doi: 10.1016/0022-2836(69)90216-2. [DOI] [PubMed] [Google Scholar]
- Bott K. F., Wilson G. A. Development of competence in the Bacillus subtilis transformation system. J Bacteriol. 1967 Sep;94(3):562–570. doi: 10.1128/jb.94.3.562-570.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. C., Doyle R. J., Streips U. N. Comparison of various procedures for removing proteins and nucleic acids from cell walls of Bacillus subtilis. Prep Biochem. 1976;6(6):479–488. doi: 10.1080/00327487608069130. [DOI] [PubMed] [Google Scholar]
- CATLIN B. W. Extracellular deoxyribonucleic acid of bacteria and a deoxyribonuclease inhibitor. Science. 1956 Sep 7;124(3219):441–442. doi: 10.1126/science.124.3219.441. [DOI] [PubMed] [Google Scholar]
- CATLIN B. W. Transformation of Neisseria meningitidis by deoxyribonucleates from cells and from culture slime. J Bacteriol. 1960 Apr;79:579–590. doi: 10.1128/jb.79.4.579-590.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. E., D'Ambrosio J., Brown N. C. Inhibition of Bacillus subtilis deoxyribonucleic acid polymerase III by phenylhydrazinopyrimidines. Demonstration of a drug-induced deoxyribonucleic acid-enzyme complex. J Biol Chem. 1975 Jan 25;250(2):522–526. [PubMed] [Google Scholar]
- Crabb W. D., Streips U. N., Doyle R. J. Selective enrichment for genetic markers in DNA released by competent cultures of Bacillus subtilis. Mol Gen Genet. 1977 Oct 20;155(2):179–183. doi: 10.1007/BF00393157. [DOI] [PubMed] [Google Scholar]
- Ephrati-Elizur E. Spontaneous transformation in Bacillus subtilis. Genet Res. 1968 Feb;11(1):83–96. doi: 10.1017/s0016672300011216. [DOI] [PubMed] [Google Scholar]
- Garrick-Silversmith L., Torriani A. Macromolecular syntheses during germination and outgrowth of Bacillus subtilis spores. J Bacteriol. 1973 May;114(2):507–516. doi: 10.1128/jb.114.2.507-516.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass K. B., Cozzarelli N. R. Further genetic and enzymological characterization of the three Bacillus subtilis deoxyribonucleic acid polymerases. J Biol Chem. 1973 Nov 25;248(22):7688–7700. [PubMed] [Google Scholar]
- Gellert M., O'Dea M. H., Itoh T., Tomizawa J. Novobiocin and coumermycin inhibit DNA supercoiling catalyzed by DNA gyrase. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4474–4478. doi: 10.1073/pnas.73.12.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynan A., Berns A. A., Dunn G., Young M., Mandelstam J. Resporulation of outgrowing Bacillus subtilis spores. J Bacteriol. 1976 Oct;128(1):8–14. doi: 10.1128/jb.128.1.8-14.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OTTOLENGHI E., HOTCHKISS R. D. Appearance of genetic transforming activity in pneumococcal cultures. Science. 1960 Oct 28;132(3435):1257–1258. [PubMed] [Google Scholar]
- SMITHIES W. R., GIBBONS N. E. The deoxyribose nucleic acid slime layer of some halophilic bacteria. Can J Microbiol. 1955 Oct;1(8):614–621. doi: 10.1139/m55-074. [DOI] [PubMed] [Google Scholar]
- Santo L. Y., Doi R. H. Ultrastructural analysis during germination and outgrowth of Bacillus subtilis spores. J Bacteriol. 1974 Oct;120(1):475–481. doi: 10.1128/jb.120.1.475-481.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. P., Iyer V. N. Competence for genetic transformation and the release of DNA from Bacillus subtilis. Biochim Biophys Acta. 1971 Feb 25;232(1):61–71. doi: 10.1016/0005-2787(71)90491-6. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streips U. N., Young F. E. Transformation in Bacillus subtilis using excreted DNA. Mol Gen Genet. 1974;133(1):47–55. doi: 10.1007/BF00268676. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI I. Genetic transformation of Bacillus subtilis by extracellular DNA. Biochem Biophys Res Commun. 1962 Jun 4;7:467–470. doi: 10.1016/0006-291x(62)90337-6. [DOI] [PubMed] [Google Scholar]
- YOUNG F. E., SPIZIZEN J. BIOCHEMICAL ASPECTS OF COMPETENCE IN THE BACILLUS SUBTILIS TRANSFORMATION SYSTEM. II. AUTOLYTIC ENZYME ACTIVITY OF CELL WALLS. J Biol Chem. 1963 Sep;238:3126–3130. [PubMed] [Google Scholar]
- YOUNG F. E., SPIZIZEN J., CRAWFORD I. P. BIOCHEMICAL ASPECTS OF COMPETENCE IN THE BACILLUS SUBTILIS TRANSFORMATION SYSTEM. I. CHEMICAL COMPOSITION OF CELL WALLS. J Biol Chem. 1963 Sep;238:3119–3125. [PubMed] [Google Scholar]


