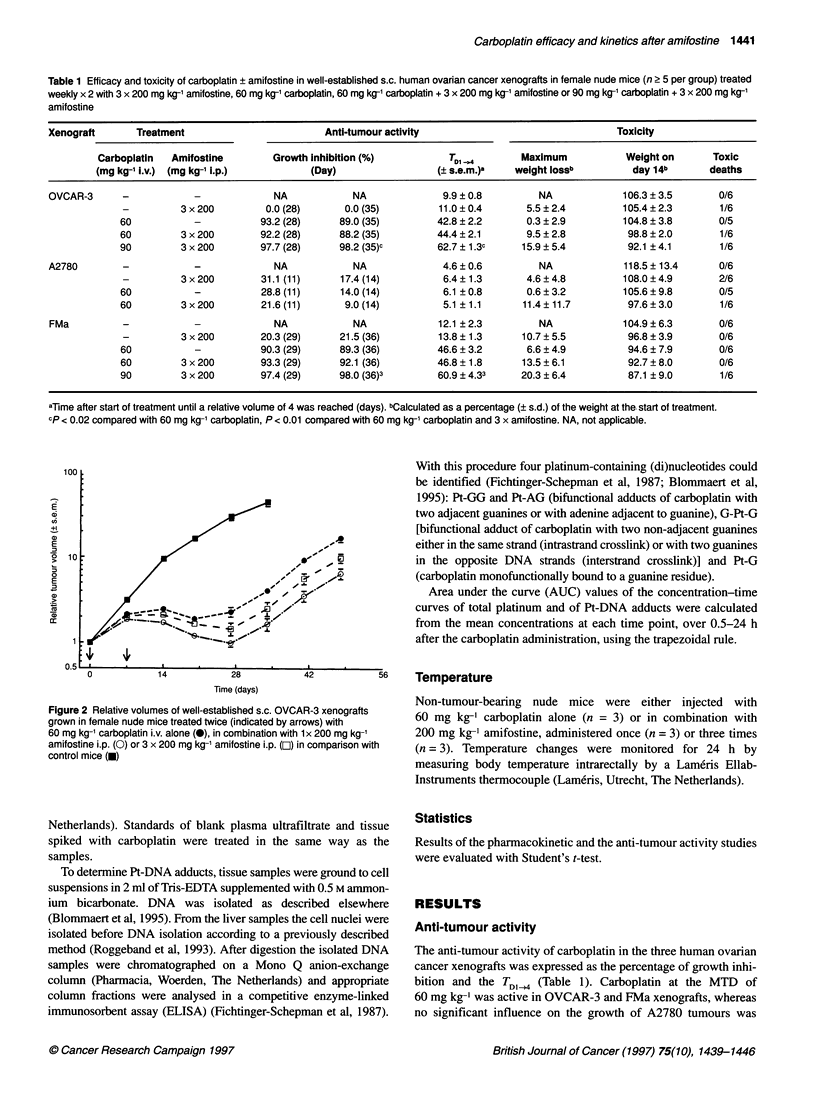
Abstract
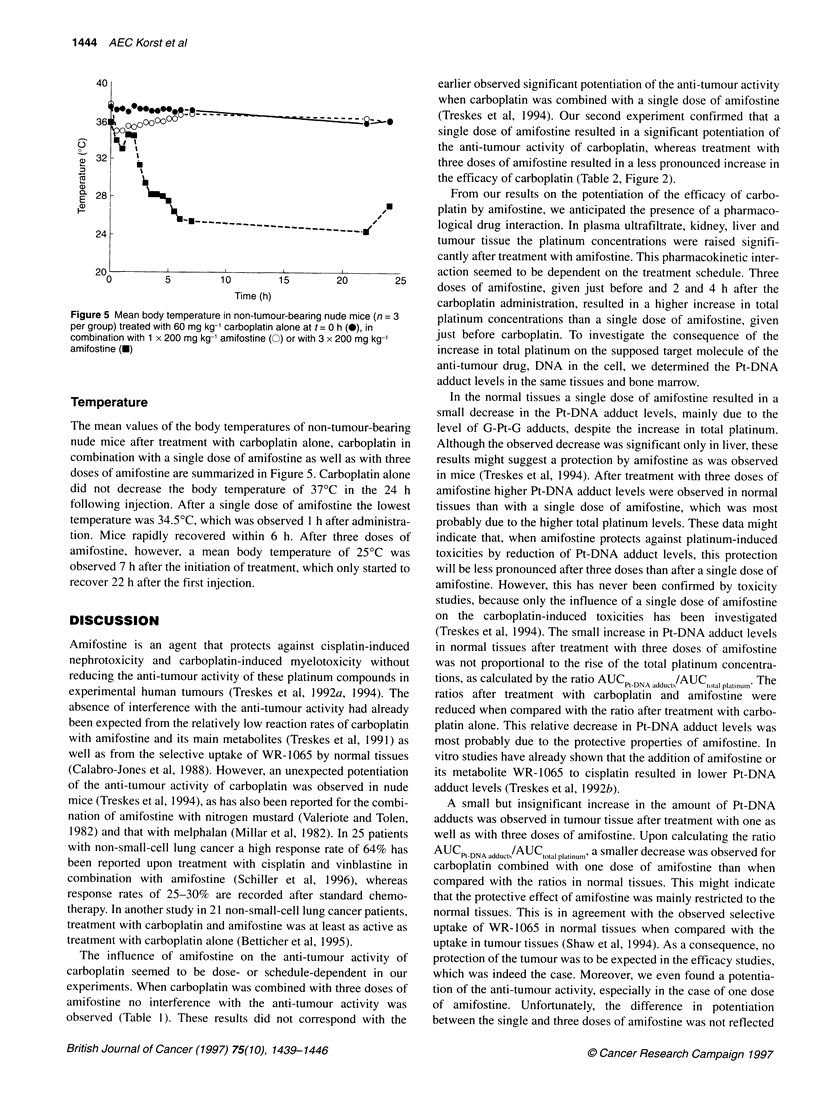
We have previously reported that amifostine potentiates the anti-tumour activity of carboplatin in mice. The present study was carried out in well-established human ovarian cancer xenografts OVCAR-3, A2780 and FMa grown subcutaneously in the nude mouse. It was found that a single dose of amifostine resulted in a higher increase in the anti-tumour activity of carboplatin than three doses of amifostine. A single dose of amifostine increased the AUC (area under the curve) values of total platinum in plasma ultrafiltrate (30.1 vs 18.2 microM x h), liver (307.7 vs 236.4 nmol g(-1) x h), kidney (500.8 vs 368.3 nmol g(-1) x h) and OVCAR-3 tumour tissue (184.0 vs 146.8 nmol g(-1) x h). Despite this increase in total platinum, a decrease in platinum (Pt)-DNA adduct levels was observed in liver, kidney and bone marrow, which was significant in liver. In tumour tissue an insignificant increase in Pt-DNA adduct levels, specifically the Pt-GG adduct, was observed after treatment with a single dose of amifostine, which may explain the increase in anti-tumour activity. The increase in the AUC of total platinum was probably caused by a reduction in body temperature, which was most severe after three doses of amifostine. The extreme hypothermia may be the reason that three doses of amifostine resulted in less potentiation of the efficacy of carboplatin.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betticher D. C., Anderson H., Ranson M., Meely K., Oster W., Thatcher N. Carboplatin combined with amifostine, a bone marrow protectant, in the treatment of non-small-cell lung cancer: a randomised phase II study. Br J Cancer. 1995 Dec;72(6):1551–1555. doi: 10.1038/bjc.1995.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blommaert F. A., van Dijk-Knijnenburg H. C., Dijt F. J., den Engelse L., Baan R. A., Berends F., Fichtinger-Schepman A. M. Formation of DNA adducts by the anticancer drug carboplatin: different nucleotide sequence preferences in vitro and in cells. Biochemistry. 1995 Jul 4;34(26):8474–8480. doi: 10.1021/bi00026a031. [DOI] [PubMed] [Google Scholar]
- Boven E., van der Vijgh W. J., Nauta M. M., Schlüper H. M., Pinedo H. M. Comparative activity and distribution studies of five platinum analogues in nude mice bearing human ovarian carcinoma xenografts. Cancer Res. 1985 Jan;45(1):86–90. [PubMed] [Google Scholar]
- Brown D. Q., Graham W. J., 3rd, MacKenzie L. J., Pittock J. W., 3rd, Shaw L. M. Can WR-2721 be improved upon? Pharmacol Ther. 1988;39(1-3):157–168. doi: 10.1016/0163-7258(88)90057-5. [DOI] [PubMed] [Google Scholar]
- Calabro-Jones P. M., Aguilera J. A., Ward J. F., Smoluk G. D., Fahey R. C. Uptake of WR-2721 derivatives by cells in culture: identification of the transported form of the drug. Cancer Res. 1988 Jul 1;48(13):3634–3640. [PubMed] [Google Scholar]
- Fichtinger-Schepman A. M., van Oosterom A. T., Lohman P. H., Berends F. cis-Diamminedichloroplatinum(II)-induced DNA adducts in peripheral leukocytes from seven cancer patients: quantitative immunochemical detection of the adduct induction and removal after a single dose of cis-diamminedichloroplatinum(II). Cancer Res. 1987 Jun 1;47(11):3000–3004. [PubMed] [Google Scholar]
- Millar J. L., McElwain T. J., Clutterbuck R. D., Wist E. A. The modification of melphalan toxicity in tumor bearing mice by s-2-(3-aminopropylamino)- ethylphosphorothioic acid (WR 2721). Am J Clin Oncol. 1982 Jun;5(3):321–328. doi: 10.1097/00000421-198206000-00015. [DOI] [PubMed] [Google Scholar]
- Page R. L., Thrall D. E., Dewhirst M. W., Meyer R. E. Whole-body hyperthermia. Rationale and potential use for cancer treatment. J Vet Intern Med. 1987 Jul-Sep;1(3):110–120. doi: 10.1111/j.1939-1676.1987.tb01998.x. [DOI] [PubMed] [Google Scholar]
- Paine G. D., Taylor C. W., Lopez M. H., Johnson C. S., Capizzi R. L. Effects of amifostine and paclitaxel on growth of human ovarian carcinoma xenografts in the severe combined immune-deficient mouse: preliminary results. Semin Oncol. 1996 Aug;23(4 Suppl 8):35–39. [PubMed] [Google Scholar]
- Roggeband R., Wolterbeek A. P., Rutten A. A., Baan R. A. Comparative 32P-postlabeling analysis of benzo[a]pyrene--DNA adducts formed in vitro upon activation of benzo[a]pyrene by human, rabbit and rodent liver microsomes. Carcinogenesis. 1993 Sep;14(9):1945–1950. doi: 10.1093/carcin/14.9.1945. [DOI] [PubMed] [Google Scholar]
- Schiller J. H., Storer B., Berlin J., Wittenkeller J., Larson M., Pharo L., Larson M., Berry W. Amifostine, cisplatin, and vinblastine in metastatic non-small-cell lung cancer: a report of high response rates and prolonged survival. J Clin Oncol. 1996 Jun;14(6):1913–1921. doi: 10.1200/JCO.1996.14.6.1913. [DOI] [PubMed] [Google Scholar]
- Shaw L. M., Bonner H. S., Brown D. Q. Metabolic pathways of WR-2721 (ethyol, amifostine) in the BALB/c mouse. Drug Metab Dispos. 1994 Nov-Dec;22(6):895–902. [PubMed] [Google Scholar]
- Shaw L. M., Glover D., Turrisi A., Brown D. Q., Bonner H. S., Norfleet A. L., Weiler C., Glick J. H., Kligerman M. M. Pharmacokinetics of WR-2721. Pharmacol Ther. 1988;39(1-3):195–201. doi: 10.1016/0163-7258(88)90061-7. [DOI] [PubMed] [Google Scholar]
- Treskes M., Boven E., van de Loosdrecht A. A., Wijffels J. F., Cloos J., Peters G. J., Pinedo H. M., van der Vijgh W. J. Effects of the modulating agent WR2721 on myelotoxicity and antitumour activity in carboplatin-treated mice. Eur J Cancer. 1994;30A(2):183–187. doi: 10.1016/0959-8049(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Treskes M., Holwerda U., Klein I., Pinedo H. M., van der Vijgh W. J. The chemical reactivity of the modulating agent WR2721 (ethiofos) and its main metabolites with the antitumor agents cisplatin and carboplatin. Biochem Pharmacol. 1991 Nov 6;42(11):2125–2130. doi: 10.1016/0006-2952(91)90347-8. [DOI] [PubMed] [Google Scholar]
- Twentyman P. R. Modification by WR 2721 of the response to chemotherapy of tumours and normal tissues in the mouse. Br J Cancer. 1983 Jan;47(1):57–63. doi: 10.1038/bjc.1983.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utley J. F., Seaver N., Newton G. L., Fahey R. C. Pharmacokinetics of WR-1065 in mouse tissue following treatment with WR-2721. Int J Radiat Oncol Biol Phys. 1984 Sep;10(9):1525–1528. doi: 10.1016/0360-3016(84)90495-4. [DOI] [PubMed] [Google Scholar]
- Valeriote F., Tolen S. Protection and potentiation of nitrogen mustard cytotoxicity by WR-2721. Cancer Res. 1982 Nov;42(11):4330–4331. [PubMed] [Google Scholar]
- Yuhas J. M. Active versus passive absorption kinetics as the basis for selective protection of normal tissues by S-2-(3-aminopropylamino)-ethylphosphorothioic acid. Cancer Res. 1980 May;40(5):1519–1524. [PubMed] [Google Scholar]
- van der Vijgh W. J. Clinical pharmacokinetics of carboplatin. Clin Pharmacokinet. 1991 Oct;21(4):242–261. doi: 10.2165/00003088-199121040-00002. [DOI] [PubMed] [Google Scholar]
- van der Vijgh W. J., Peters G. J. Protection of normal tissues from the cytotoxic effects of chemotherapy and radiation by amifostine (Ethyol): preclinical aspects. Semin Oncol. 1994 Oct;21(5 Suppl 11):2–7. [PubMed] [Google Scholar]
- van der Wilt C. L., van Laar J. A., Gyergyay F., Smid K., Peters G. J. Biochemical modification of the toxicity and the anti-tumour effect of 5-fluorouracil and cis-platinum by WR-2721 in mice. Eur J Cancer. 1992;28A(12):2017–2024. doi: 10.1016/0959-8049(92)90251-v. [DOI] [PubMed] [Google Scholar]


