Abstract
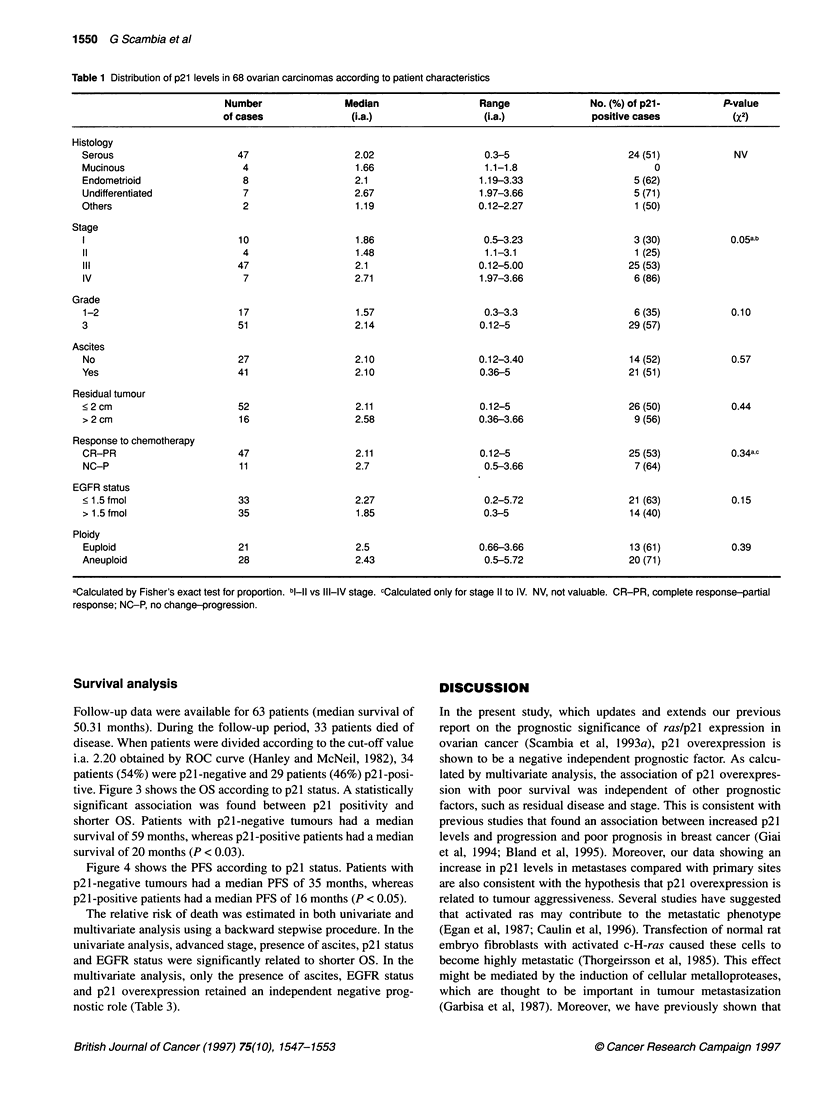
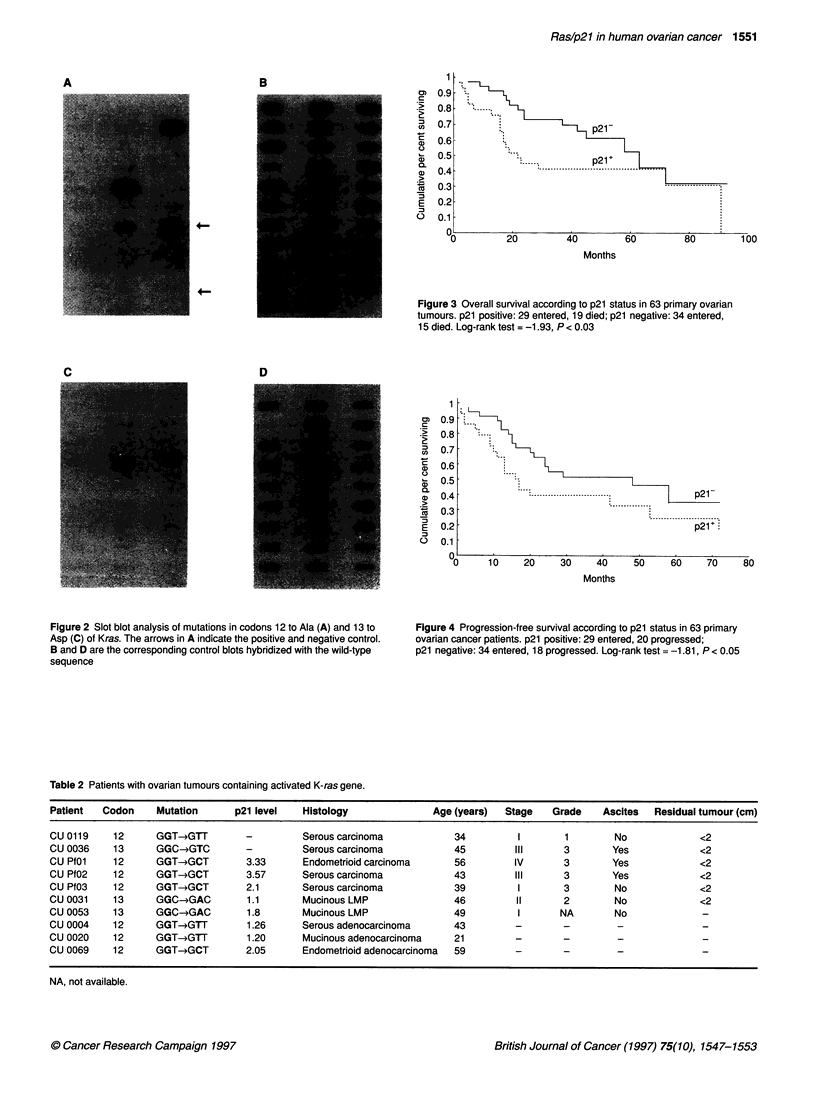
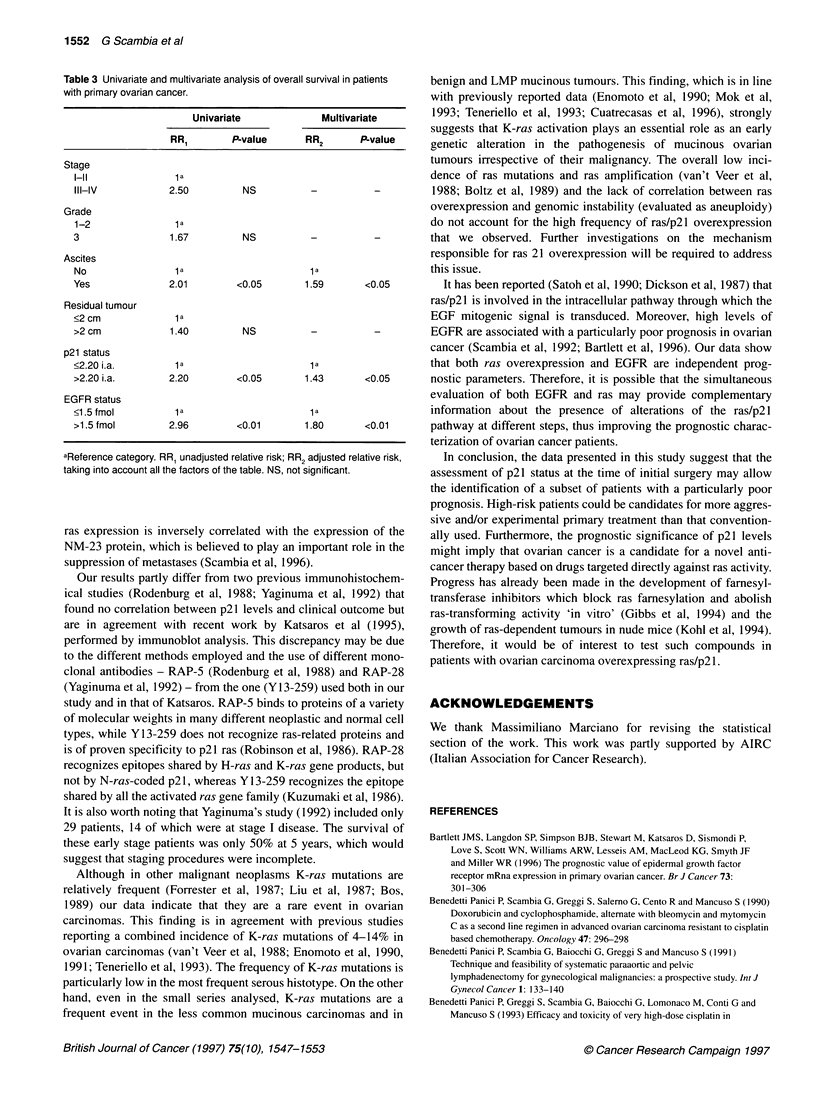
Ras/p21 oncoprotein expression and K-ras mutations were analysed by Western blot and/or K-ras oligonucleotide hybridization in 78 primary ovarian cancers, 20 omental metastases, two low malignant potential tumours (LMP), nine benign ovarian tumours and 10 normal ovaries. A cut-off value of an integral of absorbance (i.a.) of 2.20, obtained by receiver operating characteristic (ROC) curve, was shown to be the best cut-off for defining p21 positivity. p21 levels were higher in malignant tumours than in benign tumours (median 2.10 i.a. vs median 1.22 i.a.; P = 0.014) and in omental metastases than in primary ovarian carcinomas (median 2.54 i.a. vs median 2.1 i.a.; P = 0.0089). p21 overexpression did not correlate with any of the clinicopathological parameters examined. Follow-up data were available for 63 patients. A significant relationship was shown between p21 positivity and a shorter overall survival (OS) (P < 0.03) and progression-free survival (PFS) (P < 0.03). In multivariate analysis only the presence of ascites, p21 levels and epidermal growth factor receptor status retained an independent prognostic role. K-ras gene mutations were frequently detected in benign and low malignant potential tumours (71.4%), which were mostly mucinous (P = 0.0152).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett J. M., Langdon S. P., Simpson B. J., Stewart M., Katsaros D., Sismondi P., Love S., Scott W. N., Williams A. R., Lessells A. M. The prognostic value of epidermal growth factor receptor mRNA expression in primary ovarian cancer. Br J Cancer. 1996 Feb;73(3):301–306. doi: 10.1038/bjc.1996.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti Panici P., Greggi S., Scambia G., Baiocchi G., Lomonaco M., Conti G., Mancuso S. Efficacy and toxicity of very high-dose cisplatin in advanced ovarian carcinoma: 4-year survival analysis and neurological follow-up. Int J Gynecol Cancer. 1993 Jan;3(1):44–53. doi: 10.1046/j.1525-1438.1993.03010044.x. [DOI] [PubMed] [Google Scholar]
- Benedetti Panici P., Scambia G., Greggi S., Salerno G., Cento R., Mancuso S. Doxorubicin and cyclophosphamide, alternated with bleomycin and mitomycin C as a second-line regimen in advanced ovarian carcinoma resistant to cisplatin-based chemotherapy. Oncology. 1990;47(4):296–298. doi: 10.1159/000226836. [DOI] [PubMed] [Google Scholar]
- Bland K. I., Konstadoulakis M. M., Vezeridis M. P., Wanebo H. J. Oncogene protein co-expression. Value of Ha-ras, c-myc, c-fos, and p53 as prognostic discriminants for breast carcinoma. Ann Surg. 1995 Jun;221(6):706–720. doi: 10.1097/00000658-199506000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boltz E. M., Kefford R. F., Leary J. A., Houghton C. R., Friedlander M. L. Amplification of c-ras-Ki oncogene in human ovarian tumours. Int J Cancer. 1989 Mar 15;43(3):428–430. doi: 10.1002/ijc.2910430314. [DOI] [PubMed] [Google Scholar]
- Bos J. L. ras oncogenes in human cancer: a review. Cancer Res. 1989 Sep 1;49(17):4682–4689. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Caulín C., López-Barcons L., Gonzáles-Garrigues M., Navarro P., Lozano E., Rodrigo I., Gamallo C., Cano A., Fabra A., Quintanilla M. Suppression of the metastatic phenotype of a mouse skin carcinoma cell line independent of E-cadherin expression and correlated with reduced Ha-ras oncogene products. Mol Carcinog. 1996 Feb;15(2):104–114. doi: 10.1002/(SICI)1098-2744(199602)15:2<104::AID-MC3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Copeland L. J., Gershenson D. M. Ovarian cancer recurrences in patients with no macroscopic tumor at second-look laparotomy. Obstet Gynecol. 1986 Dec;68(6):873–874. [PubMed] [Google Scholar]
- Cuatrecasas M., Matias-Guiu X., Prat J. Synchronous mucinous tumors of the appendix and the ovary associated with pseudomyxoma peritonei. A clinicopathologic study of six cases with comparative analysis of c-Ki-ras mutations. Am J Surg Pathol. 1996 Jun;20(6):739–746. doi: 10.1097/00000478-199606000-00012. [DOI] [PubMed] [Google Scholar]
- Dickson R. B., Kasid A., Huff K. K., Bates S. E., Knabbe C., Bronzert D., Gelmann E. P., Lippman M. E. Activation of growth factor secretion in tumorigenic states of breast cancer induced by 17 beta-estradiol or v-Ha-ras oncogene. Proc Natl Acad Sci U S A. 1987 Feb;84(3):837–841. doi: 10.1073/pnas.84.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan S. E., Broere J. J., Jarolim L., Wright J. A., Greenberg A. H. Co-regulation of metastatic and transforming activity of normal and mutant ras genes. Int J Cancer. 1989 Mar 15;43(3):443–448. doi: 10.1002/ijc.2910430317. [DOI] [PubMed] [Google Scholar]
- Egan S. E., McClarty G. A., Jarolim L., Wright J. A., Spiro I., Hager G., Greenberg A. H. Expression of H-ras correlates with metastatic potential: evidence for direct regulation of the metastatic phenotype in 10T1/2 and NIH 3T3 cells. Mol Cell Biol. 1987 Feb;7(2):830–837. doi: 10.1128/mcb.7.2.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto T., Inoue M., Perantoni A. O., Terakawa N., Tanizawa O., Rice J. M. K-ras activation in neoplasms of the human female reproductive tract. Cancer Res. 1990 Oct 1;50(19):6139–6145. [PubMed] [Google Scholar]
- Enomoto T., Weghorst C. M., Inoue M., Tanizawa O., Rice J. M. K-ras activation occurs frequently in mucinous adenocarcinomas and rarely in other common epithelial tumors of the human ovary. Am J Pathol. 1991 Oct;139(4):777–785. [PMC free article] [PubMed] [Google Scholar]
- Forrester K., Almoguera C., Han K., Grizzle W. E., Perucho M. Detection of high incidence of K-ras oncogenes during human colon tumorigenesis. 1987 May 28-Jun 3Nature. 327(6120):298–303. doi: 10.1038/327298a0. [DOI] [PubMed] [Google Scholar]
- Garbisa S., Pozzatti R., Muschel R. J., Saffiotti U., Ballin M., Goldfarb R. H., Khoury G., Liotta L. A. Secretion of type IV collagenolytic protease and metastatic phenotype: induction by transfection with c-Ha-ras but not c-Ha-ras plus Ad2-E1a. Cancer Res. 1987 Mar 15;47(6):1523–1528. [PubMed] [Google Scholar]
- Giai M., Roagna R., Ponzone R., De Bortoli M., Dati C., Sismondi P. Prognostic and predictive relevance of c-erbB-2 and ras expression in node positive and negative breast cancer. Anticancer Res. 1994 May-Jun;14(3B):1441–1450. [PubMed] [Google Scholar]
- Gibbs J. B., Oliff A., Kohl N. E. Farnesyltransferase inhibitors: Ras research yields a potential cancer therapeutic. Cell. 1994 Apr 22;77(2):175–178. doi: 10.1016/0092-8674(94)90308-5. [DOI] [PubMed] [Google Scholar]
- Hanley J. A., McNeil B. J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982 Apr;143(1):29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- Katsaros D., Theillet C., Zola P., Louason G., Sanfilippo B., Isaia E., Arisio R., Giardina G., Sismondi P. Concurrent abnormal expression of erbB-2, myc and ras genes is associated with poor outcome of ovarian cancer patients. Anticancer Res. 1995 Jul-Aug;15(4):1501–1510. [PubMed] [Google Scholar]
- Kohl N. E., Wilson F. R., Mosser S. D., Giuliani E., deSolms S. J., Conner M. W., Anthony N. J., Holtz W. J., Gomez R. P., Lee T. J. Protein farnesyltransferase inhibitors block the growth of ras-dependent tumors in nude mice. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):9141–9145. doi: 10.1073/pnas.91.19.9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzumaki N., Oda A., Yamagiwa S., Taniguchi N., Kobayashi H., Oikawa T. Establishment of four mouse hybridoma cell lines producing monoclonal antibodies reactive with ras oncogene product p21. J Natl Cancer Inst. 1986 Dec;77(6):1273–1279. [PubMed] [Google Scholar]
- Liu E., Hjelle B., Morgan R., Hecht F., Bishop J. M. Mutations of the Kirsten-ras proto-oncogene in human preleukaemia. Nature. 1987 Nov 12;330(6144):186–188. doi: 10.1038/330186a0. [DOI] [PubMed] [Google Scholar]
- Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966 Mar;50(3):163–170. [PubMed] [Google Scholar]
- Mizuuchi H., Nasim S., Kudo R., Silverberg S. G., Greenhouse S., Garrett C. T. Clinical implications of K-ras mutations in malignant epithelial tumors of the endometrium. Cancer Res. 1992 May 15;52(10):2777–2781. [PubMed] [Google Scholar]
- Mok S. C., Bell D. A., Knapp R. C., Fishbaugh P. M., Welch W. R., Muto M. G., Berkowitz R. S., Tsao S. W. Mutation of K-ras protooncogene in human ovarian epithelial tumors of borderline malignancy. Cancer Res. 1993 Apr 1;53(7):1489–1492. [PubMed] [Google Scholar]
- O'Brien T. J., Bannon G. A., Bard D. S., Hardin J. W., Quirk J. G., Jr Expression of the ras oncogene in gynecologic tumors. Am J Obstet Gynecol. 1989 Feb;160(2):344–352. doi: 10.1016/0002-9378(89)90440-7. [DOI] [PubMed] [Google Scholar]
- Robinson A., Williams A. R., Piris J., Spandidos D. A., Wyllie A. H. Evaluation of a monoclonal antibody to ras peptide, RAP-5, claimed to bind preferentially to cells of infiltrating carcinomas. Br J Cancer. 1986 Dec;54(6):877–883. doi: 10.1038/bjc.1986.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenburg C. J., Koelma I. A., Nap M., Fleuren G. J. Immunohistochemical detection of the ras oncogene product p21 in advanced ovarian cancer. Lack of correlation with clinical outcome. Arch Pathol Lab Med. 1988 Feb;112(2):151–154. [PubMed] [Google Scholar]
- Rosell R., Li S., Skacel Z., Mate J. L., Maestre J., Canela M., Tolosa E., Armengol P., Barnadas A., Ariza A. Prognostic impact of mutated K-ras gene in surgically resected non-small cell lung cancer patients. Oncogene. 1993 Sep;8(9):2407–2412. [PubMed] [Google Scholar]
- Satoh T., Endo M., Nakafuku M., Akiyama T., Yamamoto T., Kaziro Y. Accumulation of p21ras.GTP in response to stimulation with epidermal growth factor and oncogene products with tyrosine kinase activity. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7926–7929. doi: 10.1073/pnas.87.20.7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scambia G., Benedetti Panici P., Battaglia F., Ferrandina G., Baiocchi G., Greggi S., De Vincenzo R., Mancuso S. Significance of epidermal growth factor receptor in advanced ovarian cancer. J Clin Oncol. 1992 Apr;10(4):529–535. doi: 10.1200/JCO.1992.10.4.529. [DOI] [PubMed] [Google Scholar]
- Scambia G., Benedetti Panici P., Ferrandina G., Battaglia F., Baiocchi G., Di Stefano P., Tinari N., Coronetta F., Piantelli M., Natali P. Expression of HER-2/neu oncoprotein, DNA-ploidy and S-phase fraction in advanced ovarian cancer. Int J Gynecol Cancer. 1993 Sep;3(5):271–278. doi: 10.1046/j.1525-1438.1993.03050271.x. [DOI] [PubMed] [Google Scholar]
- Scambia G., Benedetti-Panici P., Ferrandina G., Distefano M., Salerno G., Romanini M. E., Fagotti A., Mancuso S. Epidermal growth factor, oestrogen and progesterone receptor expression in primary ovarian cancer: correlation with clinical outcome and response to chemotherapy. Br J Cancer. 1995 Aug;72(2):361–366. doi: 10.1038/bjc.1995.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scambia G., Ferrandina G., Marone M., Benedetti Panici P., Giannitelli C., Piantelli M., Leone A., Mancuso S. nm23 in ovarian cancer: correlation with clinical outcome and other clinicopathologic and biochemical prognostic parameters. J Clin Oncol. 1996 Feb;14(2):334–342. doi: 10.1200/JCO.1996.14.2.334. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., deKernion J. B., Verma I. M., Cline M. J. Expression of cellular oncogenes in human malignancies. Science. 1984 Apr 20;224(4646):256–262. doi: 10.1126/science.6538699. [DOI] [PubMed] [Google Scholar]
- Teneriello M. G., Ebina M., Linnoila R. I., Henry M., Nash J. D., Park R. C., Birrer M. J. p53 and Ki-ras gene mutations in epithelial ovarian neoplasms. Cancer Res. 1993 Jul 1;53(13):3103–3108. [PubMed] [Google Scholar]
- Thorgeirsson U. P., Turpeenniemi-Hujanen T., Williams J. E., Westin E. H., Heilman C. A., Talmadge J. E., Liotta L. A. NIH/3T3 cells transfected with human tumor DNA containing activated ras oncogenes express the metastatic phenotype in nude mice. Mol Cell Biol. 1985 Jan;5(1):259–262. doi: 10.1128/mcb.5.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaginuma Y., Yamashita K., Kuzumaki N., Fujita M., Shimizu T. ras oncogene product p21 expression and prognosis of human ovarian tumors. Gynecol Oncol. 1992 Jul;46(1):45–50. doi: 10.1016/0090-8258(92)90194-n. [DOI] [PubMed] [Google Scholar]
- van 't Veer L. J., Hermens R., van den Berg-Bakker L. A., Cheng N. C., Fleuren G. J., Bos J. L., Cleton F. J., Schrier P. I. ras oncogene activation in human ovarian carcinoma. Oncogene. 1988 Feb;2(2):157–165. [PubMed] [Google Scholar]