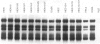Abstract
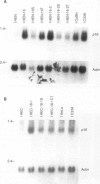
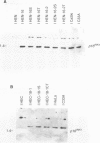
The p16 (MTS1) tumour-suppressor gene is a cyclin-dependent kinase (cdk) inhibitor that decelerates the cell cycle by inactivating the cdks that phosphorylate the retinoblastoma tumour-suppressor gene (Rb) protein (pRb). In cervical cancers, pRb is inactivated by the HPV E7 oncoprotein or by mutations. The hypothesis of earlier reports was that the disruption of the p16/cdk-cyclin/Rb cascade is essential for malignant cervical transformation/carcinogenesis. We previously established in vitro model systems of cervical cancer representing four steps of oncogenic progression initiated by the two most common oncogenic HPVs in ectocervical and endocervical epithelial cells. This report used these systems to investigate the role of p16 in cervical cancers. A dramatic enhancement of the p16 RNA level was observed after immortalization by HPV 16 or 18. Furthermore, the p16 protein was newly observed following immortalization. However, no further changes were found for RNA or protein levels after serum selection or malignant transformation. For three cervical carcinoma cell lines, similar high levels of p16 expression were seen. Point mutations or homozygous deletions of p16 were not observed in the in vitro systems or in clinical specimens. These results suggest that the inactivation of the p16/cdk-cyclin/Rb cascade does not occur during malignant transformation but occurs during the immortalization by HPV in HPV-harbouring premalignant lesions, the in situ equivalent of immortalized cells. Also suggested is that p16 has no role in the specific malignant transformation step from immortal premalignant lesions during the carcinogenesis of HPV-initiated cervical cancers.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aagaard L., Lukas J., Bartkova J., Kjerulff A. A., Strauss M., Bartek J. Aberrations of p16Ink4 and retinoblastoma tumour-suppressor genes occur in distinct sub-sets of human cancer cell lines. Int J Cancer. 1995 Mar 29;61(1):115–120. doi: 10.1002/ijc.2910610120. [DOI] [PubMed] [Google Scholar]
- Beltinger C. P., White P. S., Sulman E. P., Maris J. M., Brodeur G. M. No CDKN2 mutations in neuroblastomas. Cancer Res. 1995 May 15;55(10):2053–2055. [PubMed] [Google Scholar]
- Bosch F. X., Manos M. M., Muñoz N., Sherman M., Jansen A. M., Peto J., Schiffman M. H., Moreno V., Kurman R., Shah K. V. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995 Jun 7;87(11):796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- Brenner A. J., Aldaz C. M. Chromosome 9p allelic loss and p16/CDKN2 in breast cancer and evidence of p16 inactivation in immortal breast epithelial cells. Cancer Res. 1995 Jul 1;55(13):2892–2895. [PubMed] [Google Scholar]
- Cairns P., Mao L., Merlo A., Lee D. J., Schwab D., Eby Y., Tokino K., van der Riet P., Blaugrund J. E., Sidransky D. Rates of p16 (MTS1) mutations in primary tumors with 9p loss. Science. 1994 Jul 15;265(5170):415–417. doi: 10.1126/science.8023167. [DOI] [PubMed] [Google Scholar]
- Cairns P., Polascik T. J., Eby Y., Tokino K., Califano J., Merlo A., Mao L., Herath J., Jenkins R., Westra W. Frequency of homozygous deletion at p16/CDKN2 in primary human tumours. Nat Genet. 1995 Oct;11(2):210–212. doi: 10.1038/ng1095-210. [DOI] [PubMed] [Google Scholar]
- Caldas C., Hahn S. A., da Costa L. T., Redston M. S., Schutte M., Seymour A. B., Weinstein C. L., Hruban R. H., Yeo C. J., Kern S. E. Frequent somatic mutations and homozygous deletions of the p16 (MTS1) gene in pancreatic adenocarcinoma. Nat Genet. 1994 Sep;8(1):27–32. doi: 10.1038/ng0994-27. [DOI] [PubMed] [Google Scholar]
- Friedman L. S., Ostermeyer E. A., Szabo C. I., Dowd P., Lynch E. D., Rowell S. E., King M. C. Confirmation of BRCA1 by analysis of germline mutations linked to breast and ovarian cancer in ten families. Nat Genet. 1994 Dec;8(4):399–404. doi: 10.1038/ng1294-399. [DOI] [PubMed] [Google Scholar]
- Gu Y., Turck C. W., Morgan D. O. Inhibition of CDK2 activity in vivo by an associated 20K regulatory subunit. Nature. 1993 Dec 16;366(6456):707–710. doi: 10.1038/366707a0. [DOI] [PubMed] [Google Scholar]
- Guan K. L., Jenkins C. W., Li Y., Nichols M. A., Wu X., O'Keefe C. L., Matera A. G., Xiong Y. Growth suppression by p18, a p16INK4/MTS1- and p14INK4B/MTS2-related CDK6 inhibitor, correlates with wild-type pRb function. Genes Dev. 1994 Dec 15;8(24):2939–2952. doi: 10.1101/gad.8.24.2939. [DOI] [PubMed] [Google Scholar]
- Hannon G. J., Beach D. p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature. 1994 Sep 15;371(6494):257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- Herrington C. S. Human papillomaviruses and cervical neoplasia. II. Interaction of HPV with other factors. J Clin Pathol. 1995 Jan;48(1):1–6. doi: 10.1136/jcp.48.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirama T., Miller C. W., Wilczynski S. P., Koeffler H. P. p16 (CDKN2/cyclin-dependent kinase-4 inhibitor/multiple tumor suppressor-1) gene is not altered in uterine cervical carcinomas or cell lines. Mod Pathol. 1996 Jan;9(1):26–31. [PubMed] [Google Scholar]
- Kamb A. Cell-cycle regulators and cancer. Trends Genet. 1995 Apr;11(4):136–140. doi: 10.1016/s0168-9525(00)89027-7. [DOI] [PubMed] [Google Scholar]
- Kamb A., Gruis N. A., Weaver-Feldhaus J., Liu Q., Harshman K., Tavtigian S. V., Stockert E., Day R. S., 3rd, Johnson B. E., Skolnick M. H. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994 Apr 15;264(5157):436–440. doi: 10.1126/science.8153634. [DOI] [PubMed] [Google Scholar]
- Kelley M. J., Otterson G. A., Kaye F. J., Popescu N. C., Johnson B. E., Dipaolo J. A. CDKN2 in HPV-positive and HPV-negative cervical-carcinoma cell lines. Int J Cancer. 1995 Oct 9;63(2):226–230. doi: 10.1002/ijc.2910630214. [DOI] [PubMed] [Google Scholar]
- Koffa M., Koumantakis E., Ergazaki M., Tsatsanis C., Spandidos D. A. Association of herpesvirus infection with the development of genital cancer. Int J Cancer. 1995 Sep 27;63(1):58–62. doi: 10.1002/ijc.2910630112. [DOI] [PubMed] [Google Scholar]
- Li Y., Nichols M. A., Shay J. W., Xiong Y. Transcriptional repression of the D-type cyclin-dependent kinase inhibitor p16 by the retinoblastoma susceptibility gene product pRb. Cancer Res. 1994 Dec 1;54(23):6078–6082. [PubMed] [Google Scholar]
- Liu Q., Neuhausen S., McClure M., Frye C., Weaver-Feldhaus J., Gruis N. A., Eddington K., Allalunis-Turner M. J., Skolnick M. H., Fujimura F. K. CDKN2 (MTS1) tumor suppressor gene mutations in human tumor cell lines. Oncogene. 1995 Mar 16;10(6):1061–1067. [PubMed] [Google Scholar]
- Lukas J., Parry D., Aagaard L., Mann D. J., Bartkova J., Strauss M., Peters G., Bartek J. Retinoblastoma-protein-dependent cell-cycle inhibition by the tumour suppressor p16. Nature. 1995 Jun 8;375(6531):503–506. doi: 10.1038/375503a0. [DOI] [PubMed] [Google Scholar]
- Mitra A. B., Murty V. V., Li R. G., Pratap M., Luthra U. K., Chaganti R. S. Allelotype analysis of cervical carcinoma. Cancer Res. 1994 Aug 15;54(16):4481–4487. [PubMed] [Google Scholar]
- Mullokandov M. R., Kholodilov N. G., Atkin N. B., Burk R. D., Johnson A. B., Klinger H. P. Genomic alterations in cervical carcinoma: losses of chromosome heterozygosity and human papilloma virus tumor status. Cancer Res. 1996 Jan 1;56(1):197–205. [PubMed] [Google Scholar]
- Nakamura S., Akazawa K., Kinukawa N., Yao T., Tsuneyoshi M. Inverse correlation between the expression of bcl-2 and p53 proteins in primary gastric lymphoma. Hum Pathol. 1996 Mar;27(3):225–233. doi: 10.1016/s0046-8177(96)90061-1. [DOI] [PubMed] [Google Scholar]
- Nakao Y., Yang X., Yokoyama M., Pater M. M., Pater A. Malignant transformation of human ectocervical cells immortalized by HPV 18: in vitro model of carcinogenesis by cigarette smoke. Carcinogenesis. 1996 Mar;17(3):577–583. doi: 10.1093/carcin/17.3.577. [DOI] [PubMed] [Google Scholar]
- Noda A., Ning Y., Venable S. F., Pereira-Smith O. M., Smith J. R. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res. 1994 Mar;211(1):90–98. doi: 10.1006/excr.1994.1063. [DOI] [PubMed] [Google Scholar]
- Otterson G. A., Kratzke R. A., Coxon A., Kim Y. W., Kaye F. J. Absence of p16INK4 protein is restricted to the subset of lung cancer lines that retains wildtype RB. Oncogene. 1994 Nov;9(11):3375–3378. [PubMed] [Google Scholar]
- Pardee A. B. G1 events and regulation of cell proliferation. Science. 1989 Nov 3;246(4930):603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- Polyak K., Lee M. H., Erdjument-Bromage H., Koff A., Roberts J. M., Tempst P., Massagué J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994 Jul 15;78(1):59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- Quesnel B., Fenaux P., Philippe N., Fournier J., Bonneterre J., Preudhomme C., Peyrat J. P. Analysis of p16 gene deletion and point mutation in breast carcinoma. Br J Cancer. 1995 Aug;72(2):351–353. doi: 10.1038/bjc.1995.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A. M., Hirschhorn R. R. Expression of growth-regulated genes in normal and SV40 transformed hamster fibroblasts. J Cell Biochem. 1991 Oct;47(2):165–173. doi: 10.1002/jcb.240470210. [DOI] [PubMed] [Google Scholar]
- Sakaguchi M., Fujii Y., Hirabayashi H., Yoon H. E., Komoto Y., Oue T., Kusafuka T., Okada A., Matsuda H. Inversely correlated expression of p16 and Rb protein in non-small cell lung cancers: an immunohistochemical study. Int J Cancer. 1996 Feb 8;65(4):442–445. doi: 10.1002/(SICI)1097-0215(19960208)65:4<442::AID-IJC8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Sarma D., Yang X., Jin G., Shindoh M., Pater M. M., Pater A. Resistance to retinoic acid and altered cytokeratin expression of human papillomavirus type 16-immortalized endocervical cells after tumorigenesis. Int J Cancer. 1996 Jan 26;65(3):345–350. doi: 10.1002/(SICI)1097-0215(19960126)65:3<345::AID-IJC12>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Spruck C. H., 3rd, Gonzalez-Zulueta M., Shibata A., Simoneau A. R., Lin M. F., Gonzales F., Tsai Y. C., Jones P. A. p16 gene in uncultured tumours. Nature. 1994 Jul 21;370(6486):183–184. doi: 10.1038/370183a0. [DOI] [PubMed] [Google Scholar]
- Sreekantaiah C., Bhargava M. K., Shetty N. J. Chromosome 1 abnormalities in cervical carcinoma. Cancer. 1988 Oct 1;62(7):1317–1324. doi: 10.1002/1097-0142(19881001)62:7<1317::aid-cncr2820620713>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Sun Q., Tsutsumi K., Kelleher M. B., Pater A., Pater M. M. Squamous metaplasia of normal and carcinoma in situ of HPV 16-immortalized human endocervical cells. Cancer Res. 1992 Aug 1;52(15):4254–4260. [PubMed] [Google Scholar]
- Sun Q., Tsutsumi K., Yokoyama M., Pater M. M., Pater A. In vivo cytokeratin-expression pattern of stratified squamous epithelium from human papillomavirus-type-16-immortalized ectocervical and foreskin keratinocytes. Int J Cancer. 1993 Jun 19;54(4):656–662. doi: 10.1002/ijc.2910540422. [DOI] [PubMed] [Google Scholar]
- Tam S. W., Shay J. W., Pagano M. Differential expression and cell cycle regulation of the cyclin-dependent kinase 4 inhibitor p16Ink4. Cancer Res. 1994 Nov 15;54(22):5816–5820. [PubMed] [Google Scholar]
- Toyoshima H., Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994 Jul 15;78(1):67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- Tsutsumi K., Belaguli N., Qi S., Michalak T. I., Gulliver W. P., Pater A., Pater M. M. Human papillomavirus 16 DNA immortalizes two types of normal human epithelial cells of the uterine cervix. Am J Pathol. 1992 Feb;140(2):255–261. [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Kinzler K. W. The multistep nature of cancer. Trends Genet. 1993 Apr;9(4):138–141. doi: 10.1016/0168-9525(93)90209-z. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. The retinoblastoma protein and cell cycle control. Cell. 1995 May 5;81(3):323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- Whitaker N. J., Bryan T. M., Bonnefin P., Chang A. C., Musgrove E. A., Braithwaite A. W., Reddel R. R. Involvement of RB-1, p53, p16INK4 and telomerase in immortalisation of human cells. Oncogene. 1995 Sep 7;11(5):971–976. [PubMed] [Google Scholar]
- Xiong Y., Hannon G. J., Zhang H., Casso D., Kobayashi R., Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993 Dec 16;366(6456):701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- Yang X., Nakao Y., Pater M. M., Pater A. Identification of two novel cellular genes associated with multistage carcinogenesis of human endocervical cells by mRNA differential display. Carcinogenesis. 1996 Mar;17(3):563–567. doi: 10.1093/carcin/17.3.563. [DOI] [PubMed] [Google Scholar]
- Yeager T., Stadler W., Belair C., Puthenveettil J., Olopade O., Reznikoff C. Increased p16 levels correlate with pRb alterations in human urothelial cells. Cancer Res. 1995 Feb 1;55(3):493–497. [PubMed] [Google Scholar]
- Yokota J., Tsukada Y., Nakajima T., Gotoh M., Shimosato Y., Mori N., Tsunokawa Y., Sugimura T., Terada M. Loss of heterozygosity on the short arm of chromosome 3 in carcinoma of the uterine cervix. Cancer Res. 1989 Jul 1;49(13):3598–3601. [PubMed] [Google Scholar]
- Yokoyama M., Tsutsumi K., Pater A., Pater M. M. Human papillomavirus 18-immortalized endocervical cells with in vitro cytokeratin expression characteristics of adenocarcinoma. Obstet Gynecol. 1994 Feb;83(2):197–204. [PubMed] [Google Scholar]
- Yoshikawa H., Kawana T., Kitagawa K., Mizuno M., Yoshikura H., Iwamoto A. Detection and typing of multiple genital human papillomaviruses by DNA amplification with consensus primers. Jpn J Cancer Res. 1991 May;82(5):524–531. doi: 10.1111/j.1349-7006.1991.tb01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S. Y., Klein-Szanto A. J., Sauter E. R., Shafarenko M., Mitsunaga S., Nobori T., Carson D. A., Ridge J. A., Goodrow T. L. Higher frequency of alterations in the p16/CDKN2 gene in squamous cell carcinoma cell lines than in primary tumors of the head and neck. Cancer Res. 1994 Oct 1;54(19):5050–5053. [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- von Knebel Doeberitz M., Oltersdorf T., Schwarz E., Gissmann L. Correlation of modified human papilloma virus early gene expression with altered growth properties in C4-1 cervical carcinoma cells. Cancer Res. 1988 Jul 1;48(13):3780–3786. [PubMed] [Google Scholar]
- zur Hausen H., de Villiers E. M. Human papillomaviruses. Annu Rev Microbiol. 1994;48:427–447. doi: 10.1146/annurev.mi.48.100194.002235. [DOI] [PubMed] [Google Scholar]