Abstract
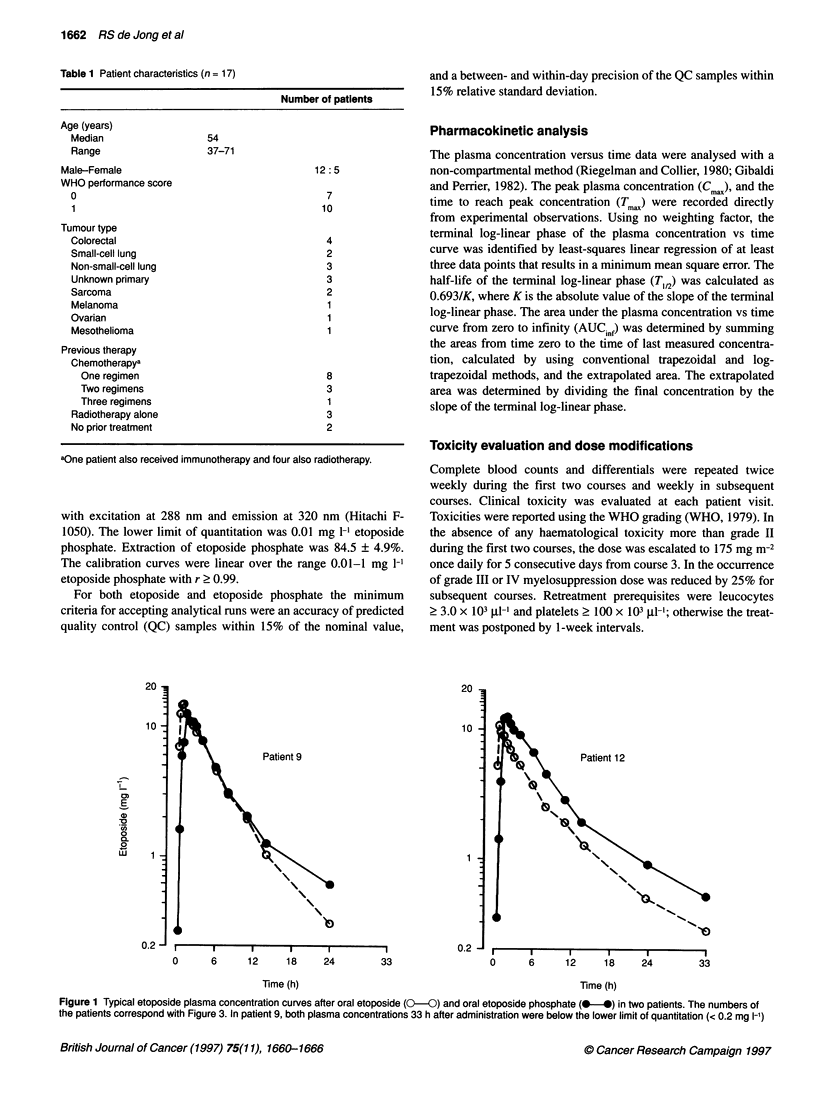
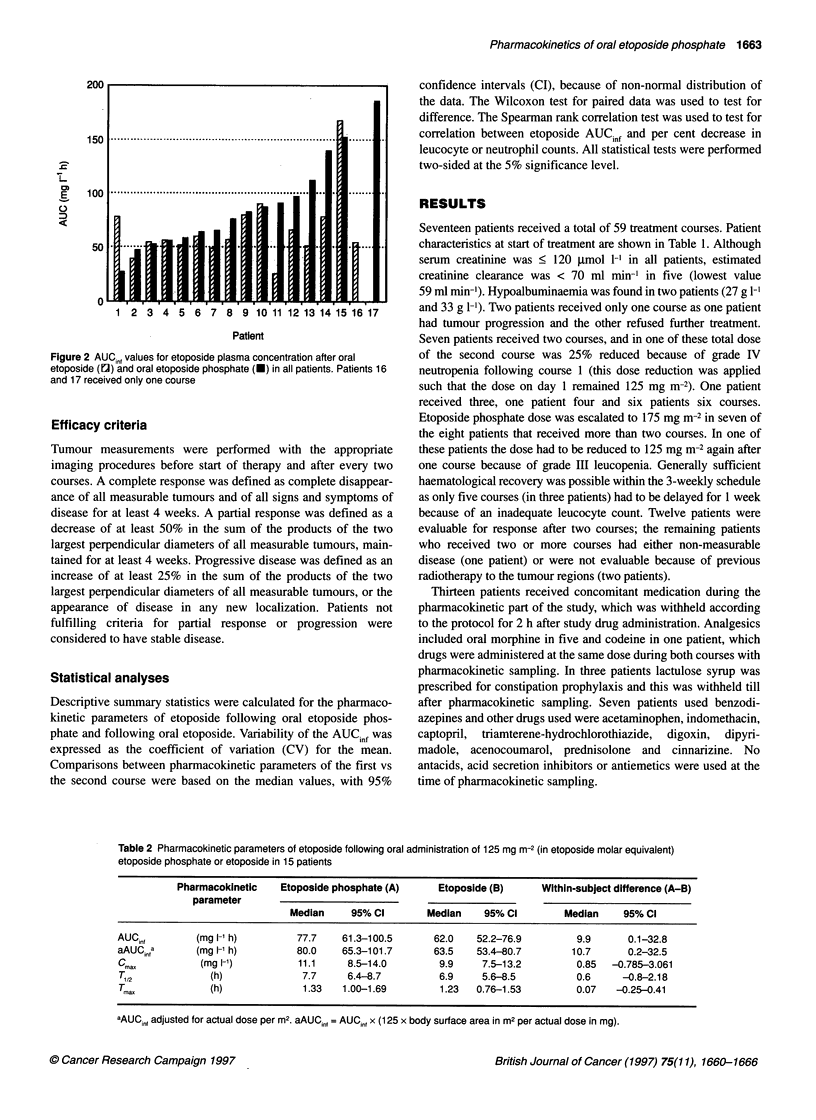
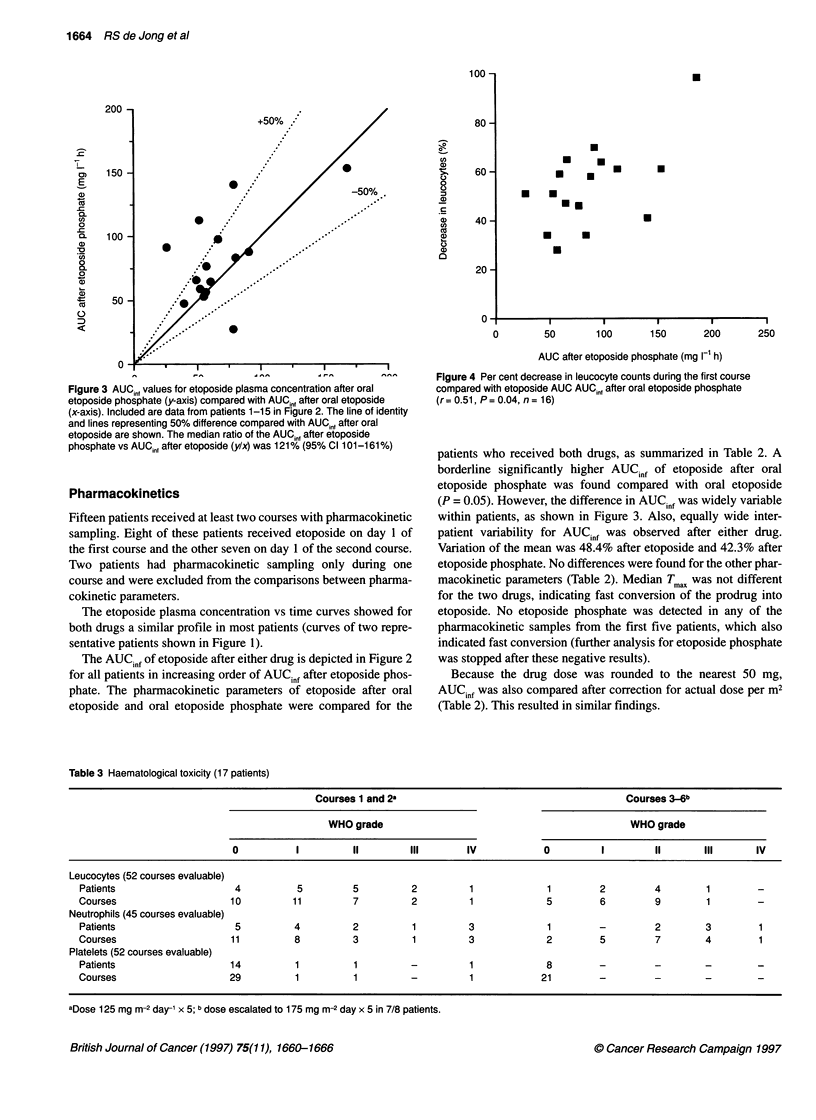
Etoposide phosphate is a water-soluble prodrug of etoposide. The plasma pharmacokinetics of etoposide following oral administration of etoposide phosphate or oral etoposide were compared. Seventeen patients with solid tumours were enrolled to receive oral etoposide phosphate 125 mg m(-2) on days 1-5 every 3 weeks, with escalation to 175 mg m(-2) from course 3 when possible. Patients were randomized to receive oral etoposide phosphate or oral etoposide on day 1 of course 1 and the alternative compound on day 1 of course 2. Fifteen patients received two or more courses and were evaluable for pharmacokinetic comparisons. The median AUC(inf) (area under the concentration vs time curve from zero to infinity) of etoposide was 77.7 mg l(-1) h after etoposide phosphate (95% CI 61.3-100.5) and 62.0 mg l(-1) h after oral etoposide (95% CI 52.2-76.9). The difference in favour of etoposide phosphate was borderline significant: median 9.9 mg l(-1) h (95% CI 0.1-32.8 mg l(-1) h; P = 0.05). However, the inter-patient variability of etoposide AUC(inf) was not improved (coefficients of variation 42.3% and 48.4%). Etoposide phosphate was undetectable in plasma after oral administration. Toxicities of oral etoposide phosphate were not different from those known for etoposide. In conclusion, oral etoposide phosphate does not offer a clinically relevant benefit over oral etoposide.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosanquet A. G., Gilby E. D. Pharmacokinetics of oral and intravenous melphalan during routine treatment of multiple myeloma. Eur J Cancer Clin Oncol. 1982 Apr;18(4):355–362. doi: 10.1016/0277-5379(82)90006-2. [DOI] [PubMed] [Google Scholar]
- Budman D. R., Igwemezie L. N., Kaul S., Behr J., Lichtman S., Schulman P., Vinciguerra V., Allen S. L., Kolitz J., Hock K. Phase I evaluation of a water-soluble etoposide prodrug, etoposide phosphate, given as a 5-minute infusion on days 1, 3, and 5 in patients with solid tumors. J Clin Oncol. 1994 Sep;12(9):1902–1909. doi: 10.1200/JCO.1994.12.9.1902. [DOI] [PubMed] [Google Scholar]
- Chabot G. G., Armand J. P., Terret C., de Forni M., Abigerges D., Winograd B., Igwemezie L., Schacter L., Kaul S., Ropers J. Etoposide bioavailability after oral administration of the prodrug etoposide phosphate in cancer patients during a phase I study. J Clin Oncol. 1996 Jul;14(7):2020–2030. doi: 10.1200/JCO.1996.14.7.2020. [DOI] [PubMed] [Google Scholar]
- Cockcroft D. W., Gault M. H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- Fields S. Z., Igwemezie L. N., Kaul S., Schacter L. P., Schilder R. J., Litam P. P., Himpler B. S., McAleer C., Wright J., Barbhaiya R. H. Phase I study of etoposide phosphate (etopophos) as a 30-minute infusion on days 1, 3, and 5. Clin Cancer Res. 1995 Jan;1(1):105–111. [PubMed] [Google Scholar]
- Hainsworth J. D., Greco F. A. Etoposide: twenty years later. Ann Oncol. 1995 Apr;6(4):325–341. doi: 10.1093/oxfordjournals.annonc.a059180. [DOI] [PubMed] [Google Scholar]
- Hainsworth J. D., Levitan N., Wampler G. L., Belani C. P., Seyedsadr M. S., Randolph J., Schacter L. P., Greco F. A. Phase II randomized study of cisplatin plus etoposide phosphate or etoposide in the treatment of small-cell lung cancer. J Clin Oncol. 1995 Jun;13(6):1436–1442. doi: 10.1200/JCO.1995.13.6.1436. [DOI] [PubMed] [Google Scholar]
- Hande K. R., Krozely M. G., Greco F. A., Hainsworth J. D., Johnson D. H. Bioavailability of low-dose oral etoposide. J Clin Oncol. 1993 Feb;11(2):374–377. doi: 10.1200/JCO.1993.11.2.374. [DOI] [PubMed] [Google Scholar]
- Harvey V. J., Slevin M. L., Joel S. P., Smythe M. M., Johnston A., Wrigley P. F. Variable bioavailability following repeated oral doses of etoposide. Eur J Cancer Clin Oncol. 1985 Nov;21(11):1315–1319. doi: 10.1016/0277-5379(85)90310-4. [DOI] [PubMed] [Google Scholar]
- Hoskins P. J., Swenerton K. D. Oral etoposide is active against platinum-resistant epithelial ovarian cancer. J Clin Oncol. 1994 Jan;12(1):60–63. doi: 10.1200/JCO.1994.12.1.60. [DOI] [PubMed] [Google Scholar]
- Liu B., Earl H. M., Poole C. J., Dunn J., Kerr D. J. Etoposide protein binding in cancer patients. Cancer Chemother Pharmacol. 1995;36(6):506–512. doi: 10.1007/BF00685801. [DOI] [PubMed] [Google Scholar]
- Martín M., Lluch A., Casado A., Santabárbara P., Adrover E., Valverde J. J., López-Martín J. A., Rodriguez-Lescure A., Azagra P., García-Conde J. Clinical activity of chronic oral etoposide in previously treated metastatic breast cancer. J Clin Oncol. 1994 May;12(5):986–991. doi: 10.1200/JCO.1994.12.5.986. [DOI] [PubMed] [Google Scholar]
- Pflüger K. H., Hahn M., Holz J. B., Schmidt L., Köhl P., Fritsch H. W., Jungclas H., Havemann K. Pharmacokinetics of etoposide: correlation of pharmacokinetic parameters with clinical conditions. Cancer Chemother Pharmacol. 1993;31(5):350–356. doi: 10.1007/BF00686147. [DOI] [PubMed] [Google Scholar]
- Riegelman S., Collier P. The application of statistical moment theory to the evaluation of in vivo dissolution time and absorption time. J Pharmacokinet Biopharm. 1980 Oct;8(5):509–534. doi: 10.1007/BF01059549. [DOI] [PubMed] [Google Scholar]
- Sessa C., Zucchetti M., Cerny T., Pagani O., Cavalli F., De Fusco M., De Jong J., Gentili D., McDaniel C., Prins C. Phase I clinical and pharmacokinetic study of oral etoposide phosphate. J Clin Oncol. 1995 Jan;13(1):200–209. doi: 10.1200/JCO.1995.13.1.200. [DOI] [PubMed] [Google Scholar]
- Slevin M. L., Clark P. I., Joel S. P., Malik S., Osborne R. J., Gregory W. M., Lowe D. G., Reznek R. H., Wrigley P. F. A randomized trial to evaluate the effect of schedule on the activity of etoposide in small-cell lung cancer. J Clin Oncol. 1989 Sep;7(9):1333–1340. doi: 10.1200/JCO.1989.7.9.1333. [DOI] [PubMed] [Google Scholar]
- Slevin M. L., Joel S. P., Whomsley R., Devenport K., Harvey V. J., Osborne R. J., Wrigley P. F. The effect of dose on the bioavailability of oral etoposide: confirmation of a clinically relevant observation. Cancer Chemother Pharmacol. 1989;24(5):329–331. doi: 10.1007/BF00304768. [DOI] [PubMed] [Google Scholar]
- Stewart C. F., Arbuck S. G., Fleming R. A., Evans W. E. Relation of systemic exposure to unbound etoposide and hematologic toxicity. Clin Pharmacol Ther. 1991 Oct;50(4):385–393. doi: 10.1038/clpt.1991.155. [DOI] [PubMed] [Google Scholar]
- Stewart C. F., Fleming R. A., Arbuck S. G., Evans W. E. Prospective evaluation of a model for predicting etoposide plasma protein binding in cancer patients. Cancer Res. 1990 Nov 1;50(21):6854–6856. [PubMed] [Google Scholar]
- Stewart C. F., Pieper J. A., Arbuck S. G., Evans W. E. Altered protein binding of etoposide in patients with cancer. Clin Pharmacol Ther. 1989 Jan;45(1):49–55. doi: 10.1038/clpt.1989.8. [DOI] [PubMed] [Google Scholar]
- Wagner T., Fenneberg K. Bioavailability of cyclophosphamide from oral formulations. Eur J Clin Pharmacol. 1984;26(2):269–270. doi: 10.1007/BF00630298. [DOI] [PubMed] [Google Scholar]
- Zimm S., Collins J. M., Riccardi R., O'Neill D., Narang P. K., Chabner B., Poplack D. G. Variable bioavailability of oral mercaptopurine. Is maintenance chemotherapy in acute lymphoblastic leukemia being optimally delivered? N Engl J Med. 1983 Apr 28;308(17):1005–1009. doi: 10.1056/NEJM198304283081705. [DOI] [PubMed] [Google Scholar]
- de Boer JF, van Rossum MC, van Albada MP, Nieuwenhuizen TM, Lagendijk A. Probability distribution of multiple scattered light measured in total transmission. Phys Rev Lett. 1994 Nov 7;73(19):2567–2570. doi: 10.1103/PhysRevLett.73.2567. [DOI] [PubMed] [Google Scholar]


