Abstract
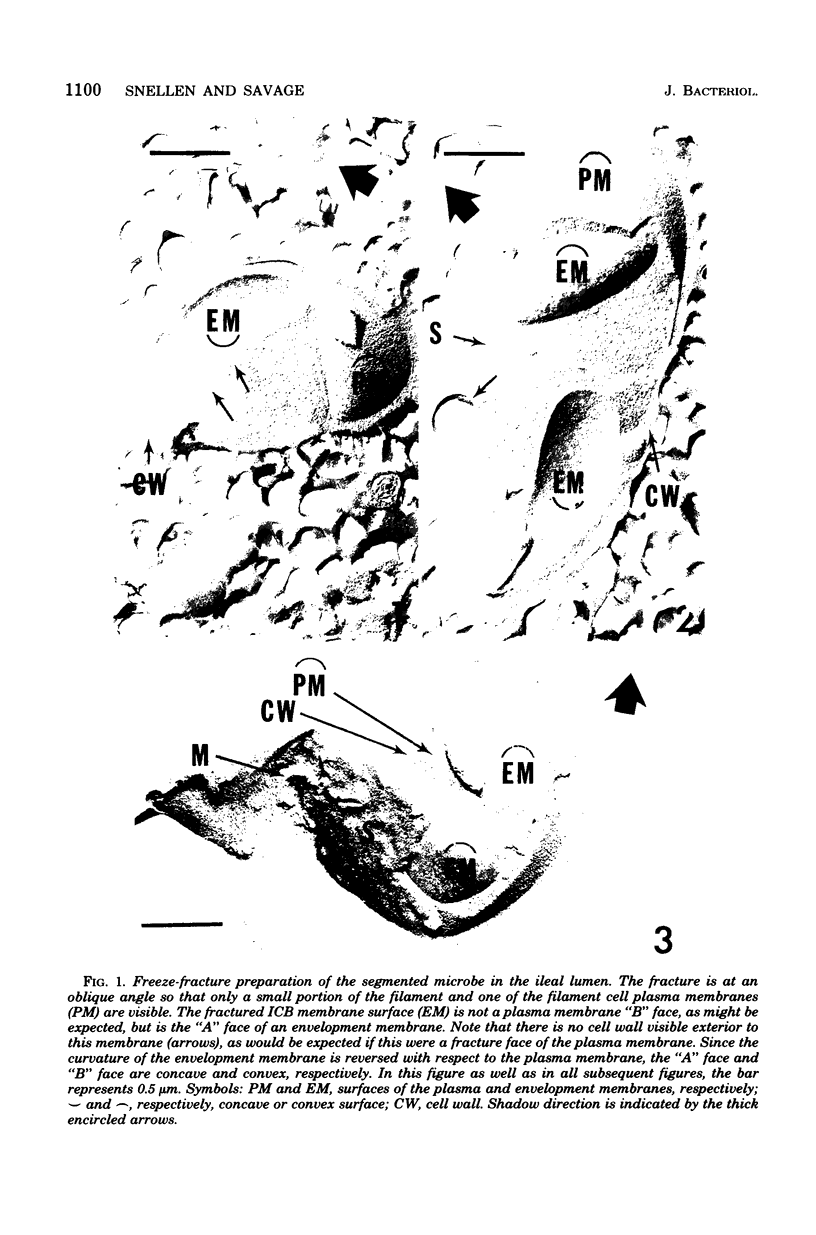
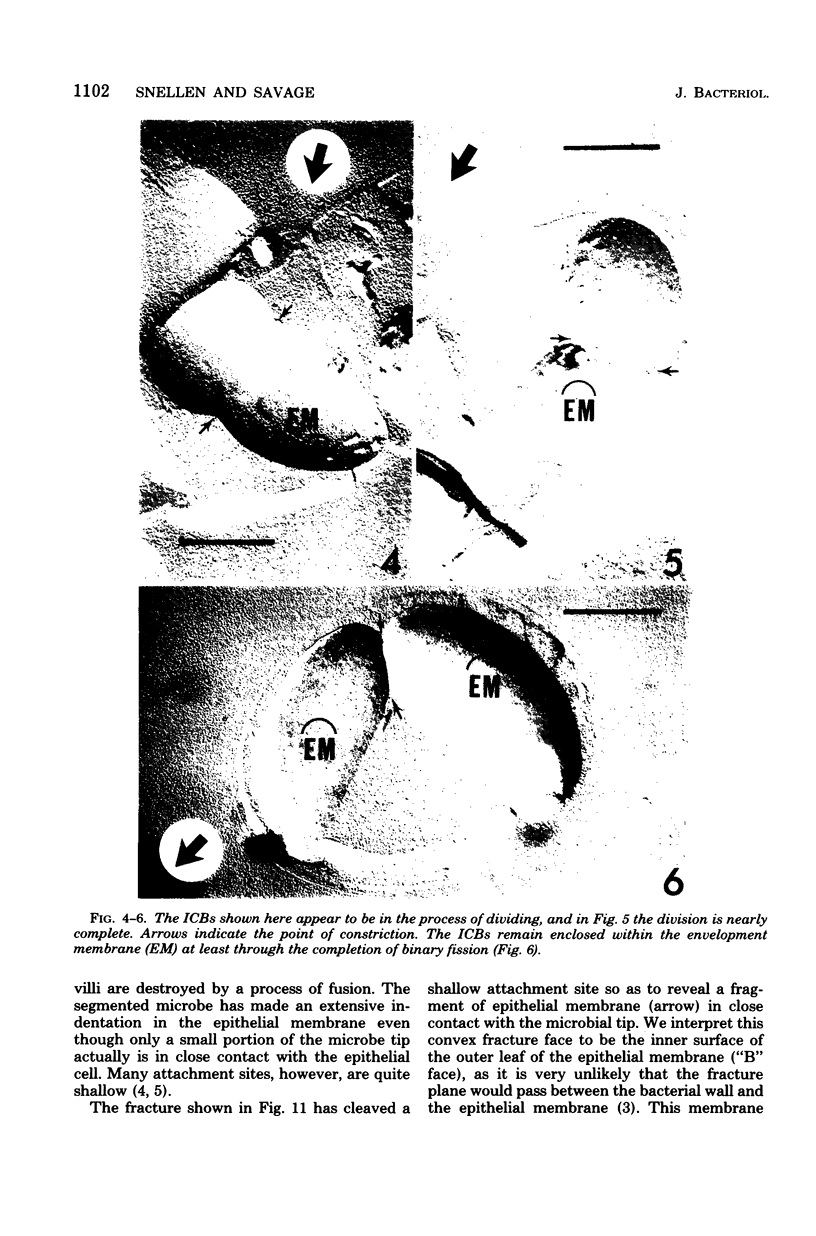
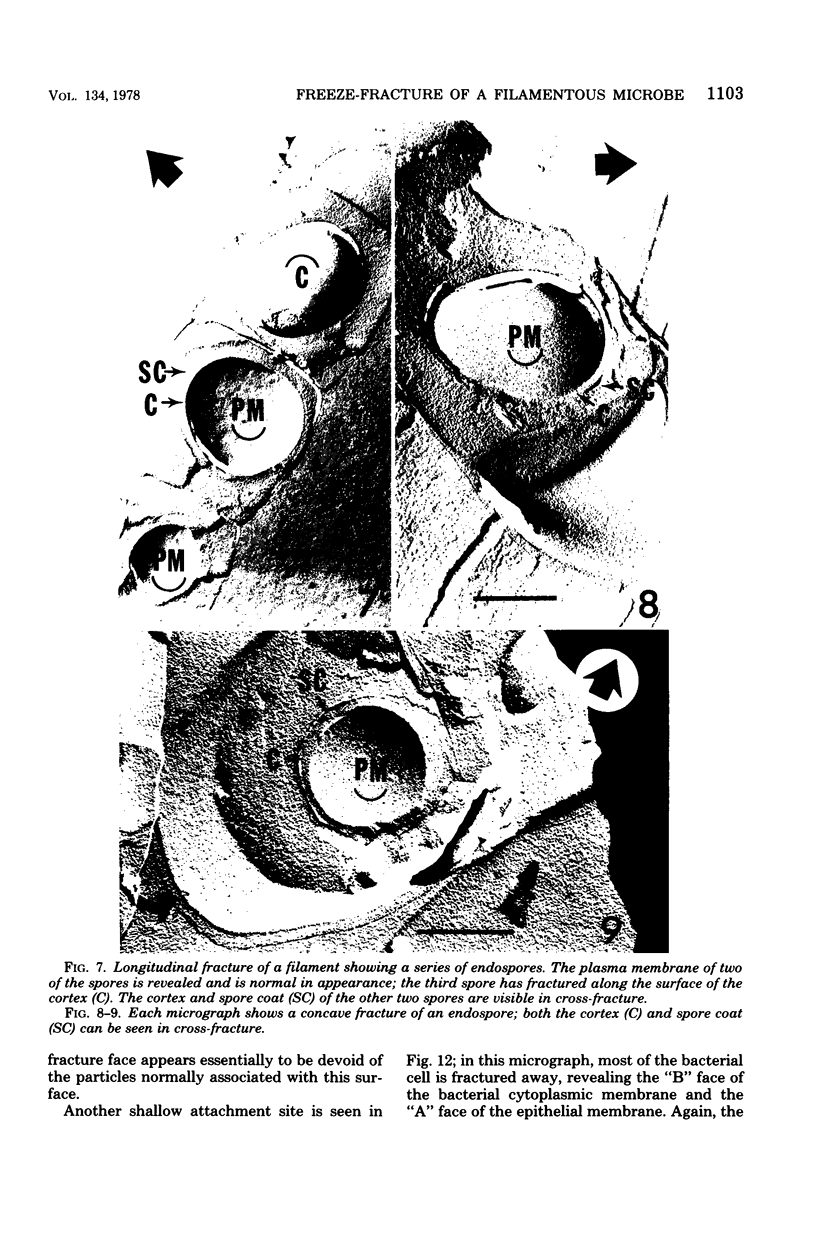
A freeze-fracture study has provided new information about the filamentous, segmented microorganism known to live in the murine small bowel. The intracellular bodies produced by this microbe appear to arise by a modified sporogenesis so that they are enclosed in an envelopment membrane at least prior to release by the filament mother cell. At least some of the intracellular bodies divide while still within the mother cell, suggesting a reproductive role for these structures. The host epithelial membrane remains intact at the site of attachment, but does appear to have a reduced concentration of intramembrane particles. Changes in the host cytoplasm adjacent to the attachment site are documented and interpreted to be a sol-gel transformation which may stabilize the attachment socket.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman M. R., Borisy G. G., Shelanski M. L., Weisenberg R. C., Taylor E. W. Cytoplasmic filaments and tubules. Fed Proc. 1968 Sep-Oct;27(5):1186–1193. [PubMed] [Google Scholar]
- Aronson A. I., Fitz-James P. Structure and morphogenesis of the bacterial spore coat. Bacteriol Rev. 1976 Jun;40(2):360–402. doi: 10.1128/br.40.2.360-402.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase D. G., Erlandsen S. L. Evidence for a complex life cycle and endospore formation in the attached, filamentous, segmented bacterium from murine ileum. J Bacteriol. 1976 Jul;127(1):572–583. doi: 10.1128/jb.127.1.572-583.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. P., Savage D. C. Effect of penicillin on the succession, attachment, and morphology of segmented, filamentous microbes in the murine small bowel. Infect Immun. 1976 Jan;13(1):180–188. doi: 10.1128/iai.13.1.180-188.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. P., Savage D. C. Habitat, succession, attachment, and morphology of segmented, filamentous microbes indigenous to the murine gastrointestinal tract. Infect Immun. 1974 Oct;10(4):948–956. doi: 10.1128/iai.10.4.948-956.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDACRE R. J., LORCH I. J. Folding and unfolding of protein molecules in relation to cytoplasmic streaming, amoeboid movement and osmotic work. Nature. 1950 Sep 23;166(4221):497–500. doi: 10.1038/166497a0. [DOI] [PubMed] [Google Scholar]
- Hampton J. C., Rosario B. The attachment of microorganisms to epithelial cells in the distal ileum of the mouse. Lab Invest. 1965 Aug;14(8):1464–1481. [PubMed] [Google Scholar]
- KOPAC M. J. Physical properties of protoplasm. Annu Rev Physiol. 1950;12:7–26. doi: 10.1146/annurev.ph.12.030150.000255. [DOI] [PubMed] [Google Scholar]
- Rodewald R., Newman S. B., Karnovsky M. J. Contraction of isolated brush borders from the intestinal epithelium. J Cell Biol. 1976 Sep;70(3):541–554. doi: 10.1083/jcb.70.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C., Blumershine R. V. Surface-surface associations in microbial communities populating epithelial habitats in the murine gastrointestinal ecosystem: scanning electron microscopy. Infect Immun. 1974 Jul;10(1):240–250. doi: 10.1128/iai.10.1.240-250.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C. Localization of certain indigenous microorganisms on the ileal villi of rats. J Bacteriol. 1969 Mar;97(3):1505–1506. doi: 10.1128/jb.97.3.1505-1506.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]