Abstract
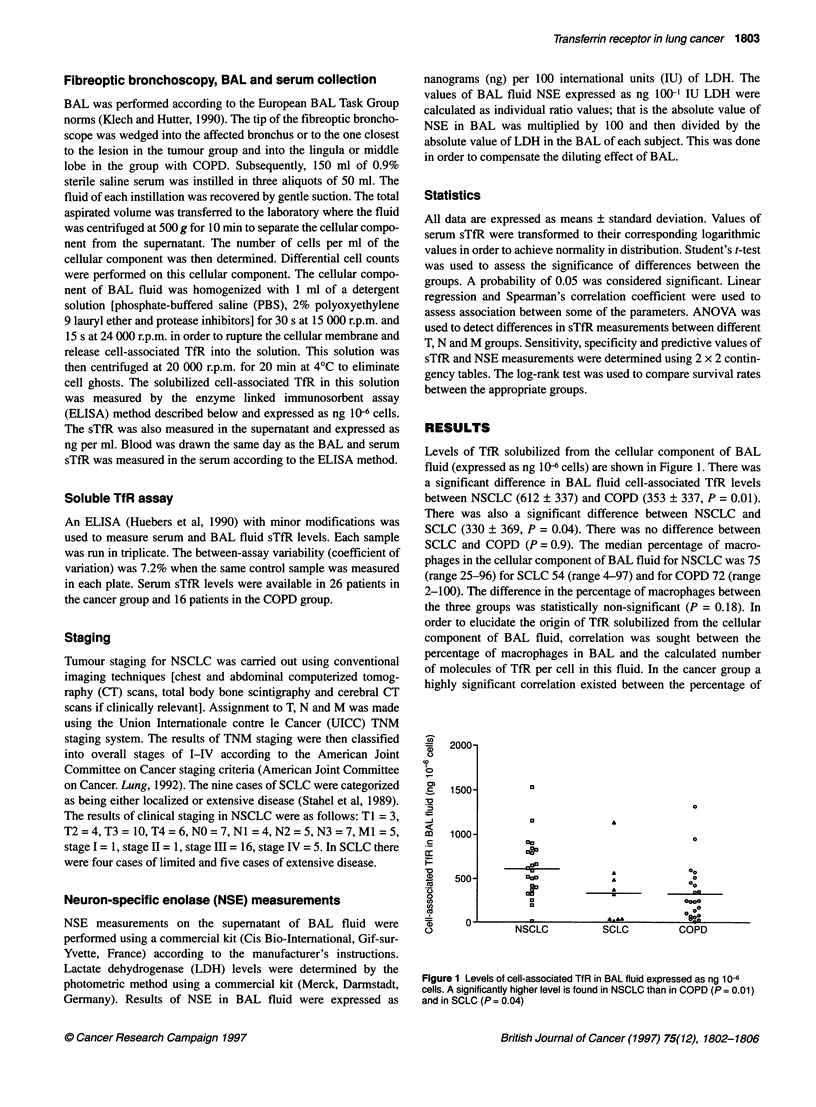
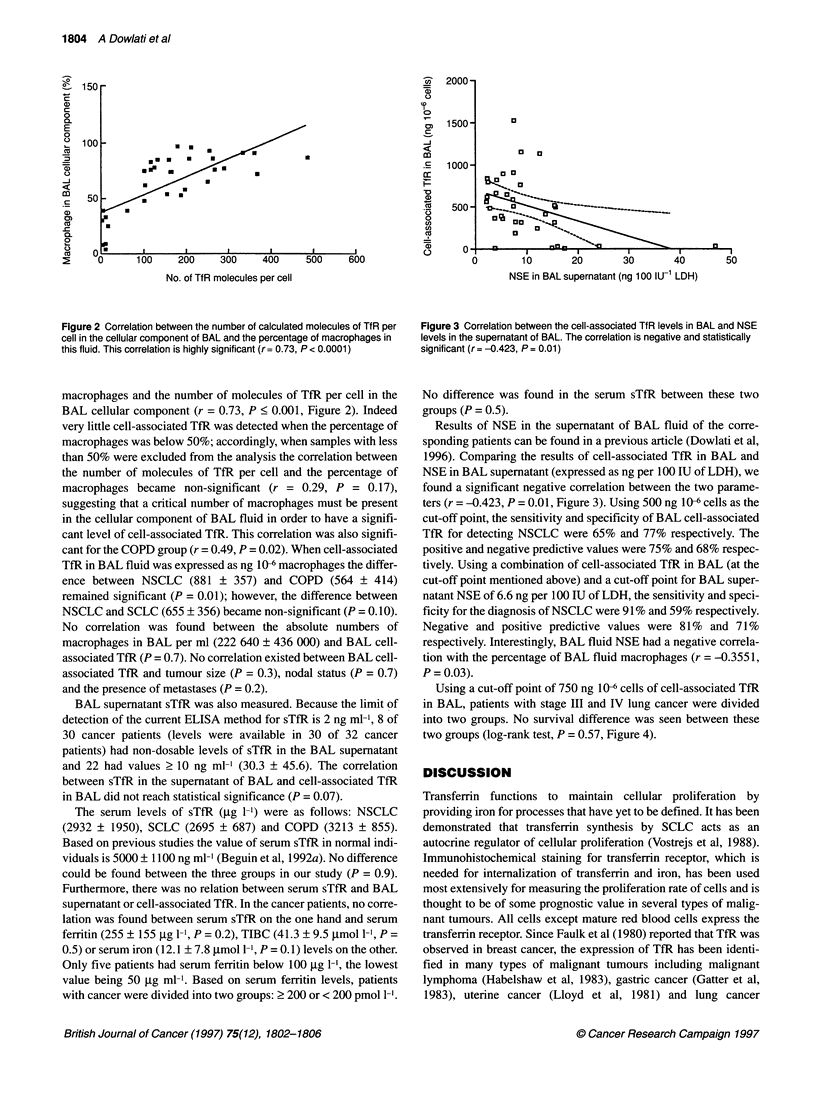
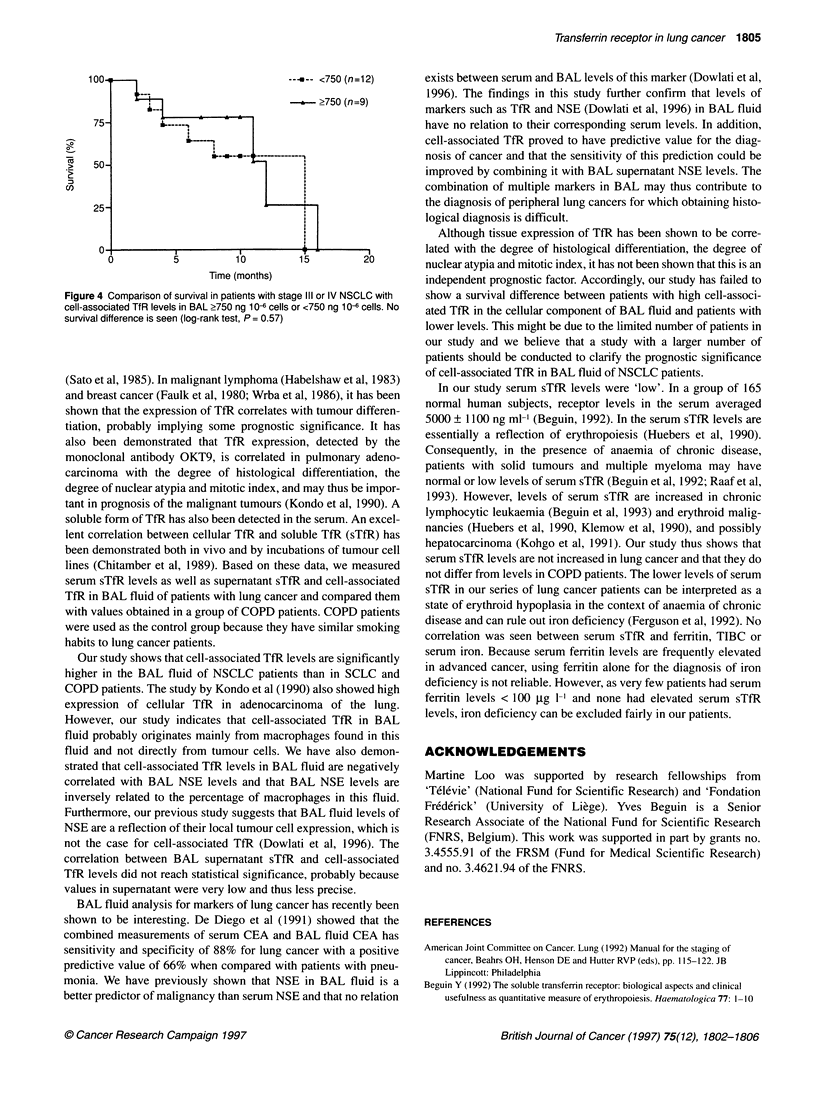
The expression of transferrin receptor (TfR) has been identified in many malignant tumours. In lung cancer, lymphoma and breast cancer, it has been shown that the expression of TfR correlates with tumour differentiation, probably implying some prognostic value. A soluble form of TfR (sTfR) in human serum has been shown to be proportional to the number of cellular TfRs. Based on these data we examined the utility of measuring sTfR in the serum and bronchoalveolar lavage (BAL) fluid of patients with lung cancer (n = 32) and patients with chronic obstructive pulmonary disease (n = 22). BAL fluid was centrifuged to separate the supernatant from the cellular component. Cells were lysed in a detergent and cell-associated TfR was measured by enzyme-linked immunosorbent assay (ELISA) and expressed as ng 10(-6) cells in this cellular component. There was no difference in serum sTfR between the cancer and chronic obstructive pulmonary disease (COPD) groups. A higher level of cell-associated TfR was found in BAL of non-small-cell lung cancer patients than in COPD patients (P = 0.01). The calculated number of TfR molecules per cell in BAL correlated positively with the percentage of macrophages in BAL (P < 0.0001), suggesting that cell-associated TfR in BAL originates primarily from macrophages in this fluid. No correlation existed between BAL cell-associated TfR and tumour size, nodal status, the presence of metastases and serum sTfR. BAL cell-associated TfR was negatively correlated with BAL supernatant neuron-specific enolase (NSE) (P = 0.01). A combination of BAL supernatant NSE and cell-associated TfR detected lung cancer with a sensitivity of 91%, a specificity of 59% and positive and negative predictive values of 81% and 71% respectively. In conclusion, BAL cell-associated TfR may help in the differential diagnosis of lung cancer vs pneumonia.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beguin Y., Lampertz S., De Groote D., Igot D., Malaise M., Fillet G. Soluble CD23 and other receptors (CD4, CD8, CD25, CD71) in serum of patients with chronic lymphocytic leukemia. Leukemia. 1993 Dec;7(12):2019–2025. [PubMed] [Google Scholar]
- Beguin Y. The soluble transferrin receptor: biological aspects and clinical usefulness as quantitative measure of erythropoiesis. Haematologica. 1992 Jan-Feb;77(1):1–10. [PubMed] [Google Scholar]
- Beguin Y., Yerna M., Loo M., Weber M., Fillet G. Erythropoiesis in multiple myeloma: defective red cell production due to inappropriate erythropoietin production. Br J Haematol. 1992 Dec;82(4):648–653. doi: 10.1111/j.1365-2141.1992.tb06939.x. [DOI] [PubMed] [Google Scholar]
- Chitambar C. R., Zivkovic Z. Release of soluble transferrin receptor from the surface of human leukemic HL60 cells. Blood. 1989 Aug 1;74(2):602–608. [PubMed] [Google Scholar]
- Clinical guidelines and indications for bronchoalveolar lavage (BAL): Report of the European Society of Pneumology Task Group on BAL. Eur Respir J. 1990 Sep;3(8):937–976. [PubMed] [Google Scholar]
- Dowlati A., Bury T., Corhay J. L., Weber T., Mendes P., Radermecker M. High neuron specific enolase levels in bronchoalveolar lavage fluid of patients with lung carcinoma: diagnostic value, relation to serum neuron specific enolase, and staging. Cancer. 1996 May 15;77(10):2039–2043. doi: 10.1002/(SICI)1097-0142(19960515)77:10<2039::AID-CNCR11>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Faulk W. P., Hsi B. L., Stevens P. J. Transferrin and transferrin receptors in carcinoma of the breast. Lancet. 1980 Aug 23;2(8191):390–392. doi: 10.1016/s0140-6736(80)90440-7. [DOI] [PubMed] [Google Scholar]
- Ferguson B. J., Skikne B. S., Simpson K. M., Baynes R. D., Cook J. D. Serum transferrin receptor distinguishes the anemia of chronic disease from iron deficiency anemia. J Lab Clin Med. 1992 Apr;119(4):385–390. [PubMed] [Google Scholar]
- Gatter K. C., Brown G., Trowbridge I. S., Woolston R. E., Mason D. Y. Transferrin receptors in human tissues: their distribution and possible clinical relevance. J Clin Pathol. 1983 May;36(5):539–545. doi: 10.1136/jcp.36.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeshaw J. A., Lister T. A., Stansfeld A. G., Greaves M. F. Correlation of transferrin receptor expression with histological class and outcome in non-Hodgkin lymphoma. Lancet. 1983 Mar 5;1(8323):498–501. doi: 10.1016/s0140-6736(83)92191-8. [DOI] [PubMed] [Google Scholar]
- Huebers H. A., Beguin Y., Pootrakul P., Einspahr D., Finch C. A. Intact transferrin receptors in human plasma and their relation to erythropoiesis. Blood. 1990 Jan 1;75(1):102–107. [PubMed] [Google Scholar]
- Klemow D., Einsphar D., Brown T. A., Flowers C. H., Skikne B. S. Serum transferrin receptor measurements in hematologic malignancies. Am J Hematol. 1990 Jul;34(3):193–198. doi: 10.1002/ajh.2830340307. [DOI] [PubMed] [Google Scholar]
- Kohgo Y., Kondo H., Mogi Y., Niitsu Y. Mechanism and clinical significance of soluble hepatic cell-surface receptors. Targeted Diagn Ther. 1991;4:305–319. [PubMed] [Google Scholar]
- Kondo K., Noguchi M., Mukai K., Matsuno Y., Sato Y., Shimosato Y., Monden Y. Transferrin receptor expression in adenocarcinoma of the lung as a histopathologic indicator of prognosis. Chest. 1990 Jun;97(6):1367–1371. doi: 10.1378/chest.97.6.1367. [DOI] [PubMed] [Google Scholar]
- Lloyd J. M., O'Dowd T., Driver M., Tee D. E. Demonstration of an epitope of the transferrin receptor in human cervical epithelium--a potentially useful cell marker. J Clin Pathol. 1984 Feb;37(2):131–135. doi: 10.1136/jcp.37.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaf H. N., Jacobsen D. W., Savon S., Green R. Serum transferrin receptor level is not altered in invasive adenocarcinoma of the breast. Am J Clin Pathol. 1993 Mar;99(3):232–237. doi: 10.1093/ajcp/99.3.232. [DOI] [PubMed] [Google Scholar]
- Sato Y., Watanabe S., Kodama T., Goto M., Shimosato Y. Stainability of lung cancer cells with Leu-7 and OKT-9 monoclonal antibodies. Jpn J Clin Oncol. 1985 Sep;15(3):537–544. [PubMed] [Google Scholar]
- Vostrejs M., Moran P. L., Seligman P. A. Transferrin synthesis by small cell lung cancer cells acts as an autocrine regulator of cellular proliferation. J Clin Invest. 1988 Jul;82(1):331–339. doi: 10.1172/JCI113591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrba F., Ritzinger E., Reiner A., Holzner J. H. Transferrin receptor (TrfR) expression in breast carcinoma and its possible relationship to prognosis. An immunohistochemical study. Virchows Arch A Pathol Anat Histopathol. 1986;410(1):69–73. doi: 10.1007/BF00710908. [DOI] [PubMed] [Google Scholar]
- de Diego A., Compte L., Sanchis J., Enguidanos M. J., Marco V. Usefulness of carcinoembryonic antigen determination in bronchoalveolar lavage fluid. A comparative study among patients with peripheral lung cancer, pneumonia, and healthy individuals. Chest. 1991 Oct;100(4):1060–1063. doi: 10.1378/chest.100.4.1060. [DOI] [PubMed] [Google Scholar]


