Abstract
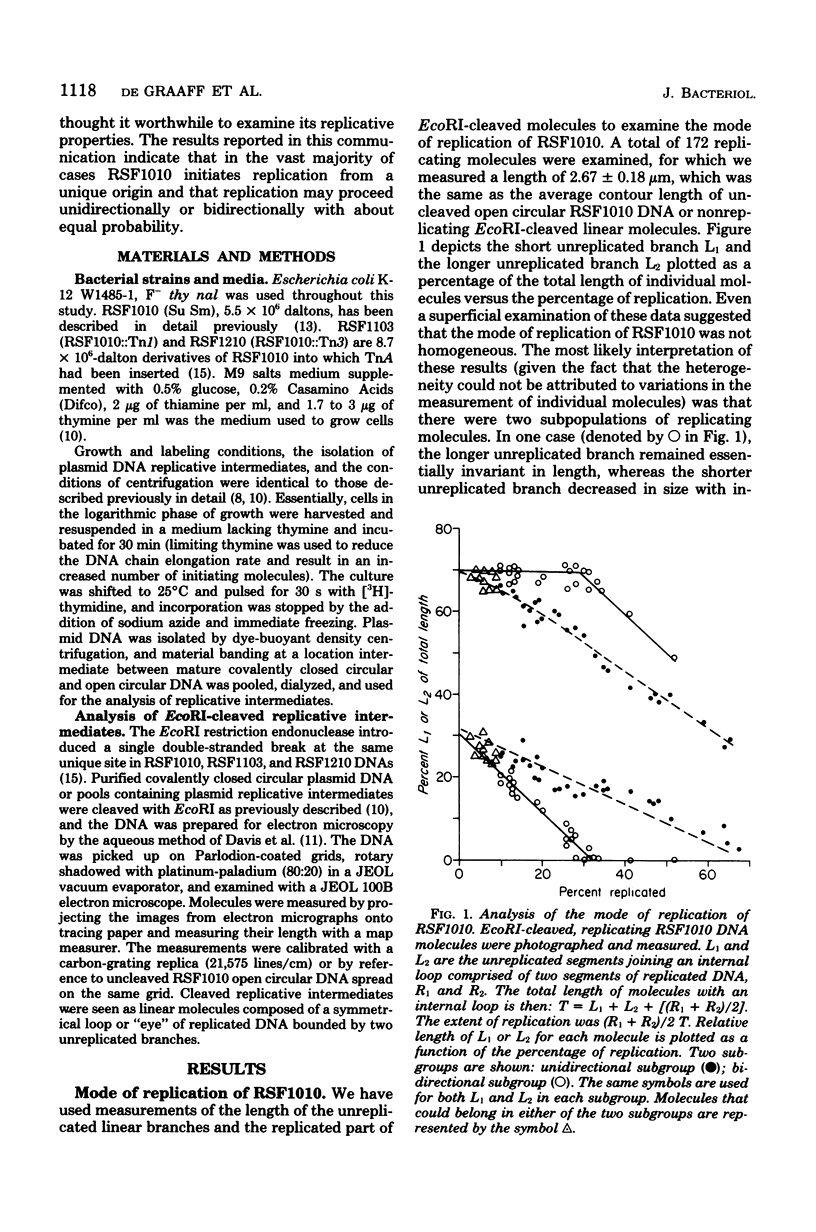
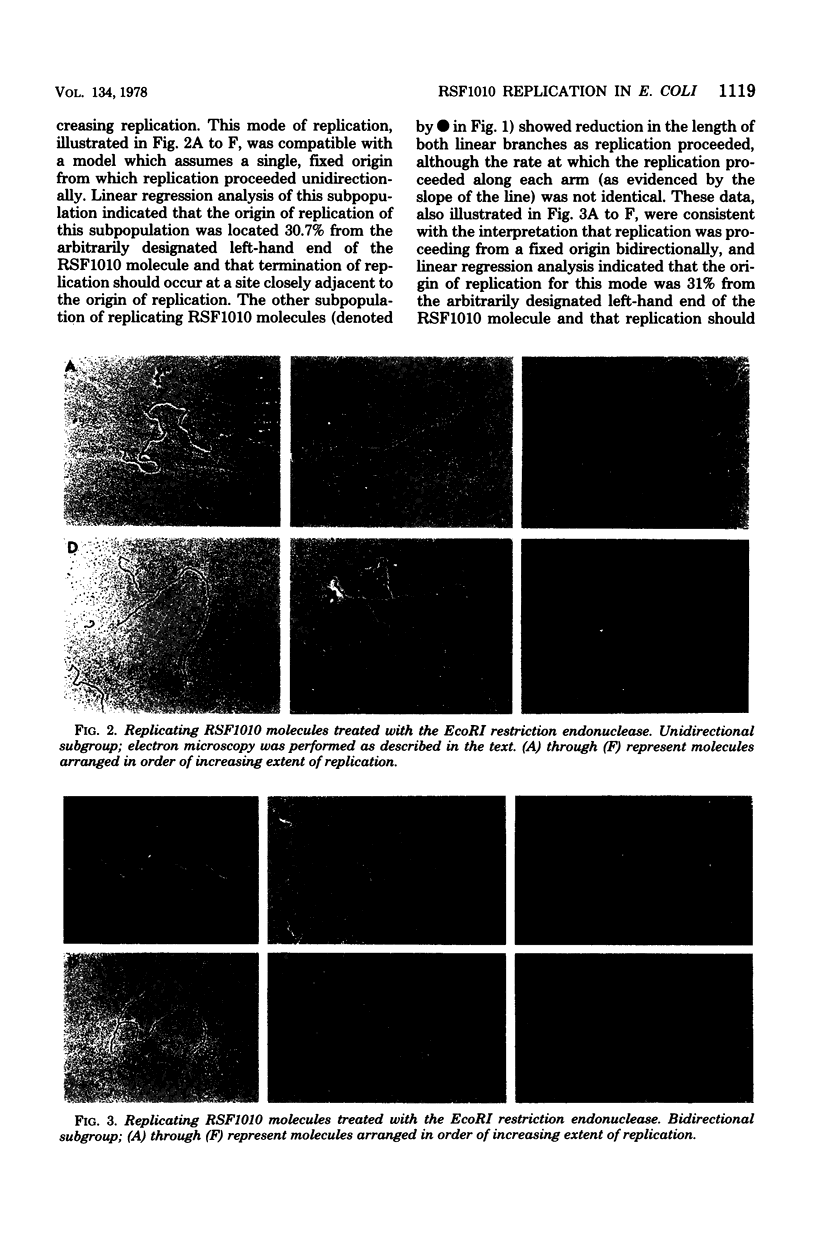
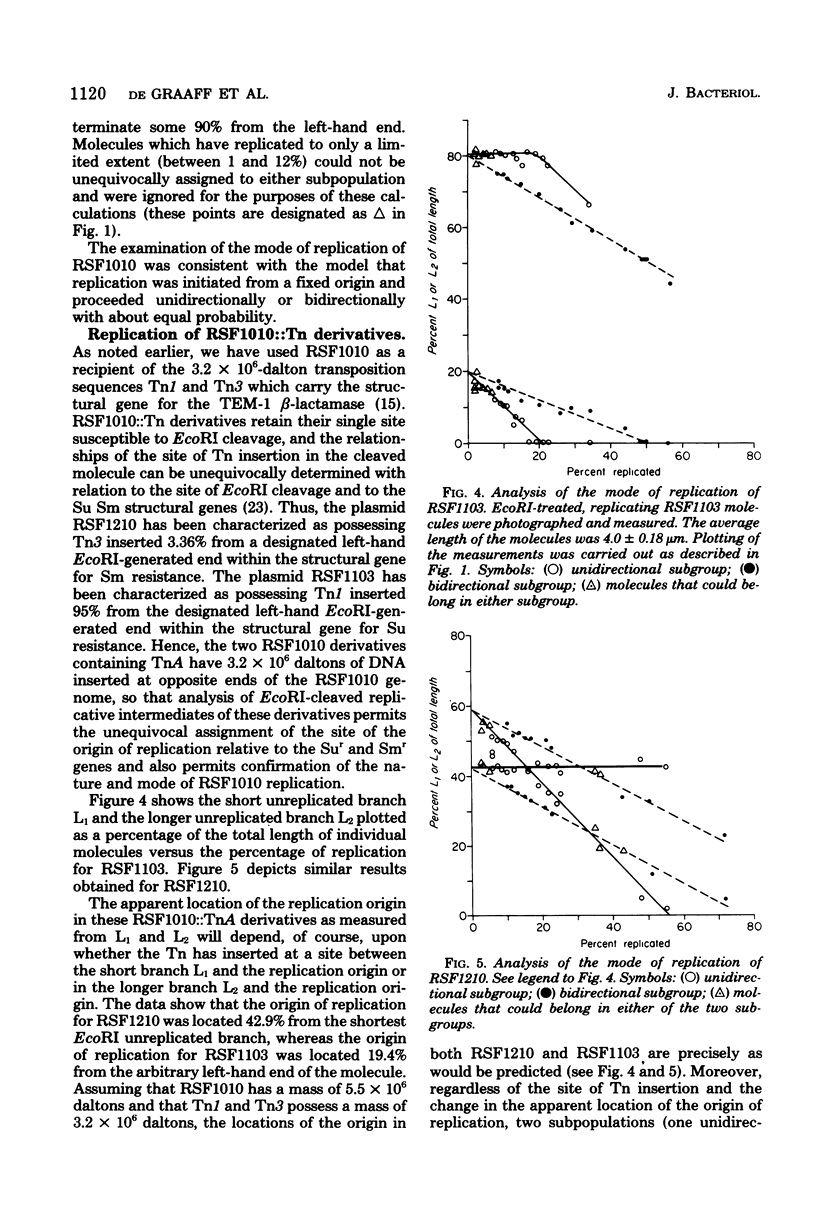
Replicating DNA molecules of the nonconjugative R plasmid RSF1010 (Smr Sur) were cleaved with the EcoRI restriction endonuclease and examined with the electron microscope. Results of this analysis indicated that replication is initiated from an origin located at about 19% of total genome size from one of the EcoRI ends. Replication proceeded either unidirectionally or bidirectionally with equal frequency. Results of the analysis of replicative intermediates of RSF1010 containing the Apr-transposable sequence (Tn) are also presented.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. S., Lewis M. J. Characterization of a transfer factor associated with drug resistance in Salmonella typhimurium. Nature. 1965 Nov 27;208(5013):843–849. doi: 10.1038/208843a0. [DOI] [PubMed] [Google Scholar]
- Anderson E. S., Lewis M. J. Drug resistance and its transfer in Salmonella typhimurium. Nature. 1965 May 8;206(984):579–583. doi: 10.1038/206579a0. [DOI] [PubMed] [Google Scholar]
- Anderson E. S. The ecology of transferable drug resistance in the enterobacteria. Annu Rev Microbiol. 1968;22:131–180. doi: 10.1146/annurev.mi.22.100168.001023. [DOI] [PubMed] [Google Scholar]
- Barth P. T., Grinter N. J. Comparison of the deoxyribonucleic acid molecular weights and homologies of plasmids conferring linked resistance to streptomycin and sulfonamides. J Bacteriol. 1974 Nov;120(2):618–630. doi: 10.1128/jb.120.2.618-630.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Clayton D. A. Mechanism of mitochondrial DNA replication in mouse L-cells: topology of circular daughter molecules and dynamics of catenated oligomer formation. J Mol Biol. 1976 Jan 5;100(1):85–92. doi: 10.1016/s0022-2836(76)80036-8. [DOI] [PubMed] [Google Scholar]
- Bourgaux P., Bourgaux-Ramoisy D. Unwinding of replicating polyoma virus DNA. J Mol Biol. 1972 Oct 14;70(3):399–413. doi: 10.1016/0022-2836(72)90548-7. [DOI] [PubMed] [Google Scholar]
- Cabello F., Timmis K., Cohen S. N. Replication control in a composite plasmid constructed by in vitro linkage of two distinct replicons. Nature. 1976 Jan 29;259(5541):285–290. doi: 10.1038/259285a0. [DOI] [PubMed] [Google Scholar]
- Crosa J. H., Luttropp L. K., Falkow S. Mode of replication of the conjugative R-plasmid RSF1040 in Escherichia coli. J Bacteriol. 1976 Apr;126(1):454–466. doi: 10.1128/jb.126.1.454-466.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H., Luttropp L. K., Heffron F., Falkow S. Two replication initiation sites on R-plasmid DNA. Mol Gen Genet. 1975 Sep 15;140(1):39–50. doi: 10.1007/BF00268987. [DOI] [PubMed] [Google Scholar]
- Eichenlaub R., Figurski D., Helinski D. R. Bidirection replication from a unique origin in a mini-F plasmid. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1138–1141. doi: 10.1073/pnas.74.3.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindley N. D., Kelley W. S. Effects of different alleles of the E. coli K12 pol A gene on the replication of non-transferring plasmids. Mol Gen Genet. 1976 Feb 2;143(3):311–318. doi: 10.1007/BF00269409. [DOI] [PubMed] [Google Scholar]
- Guerry P., van Embden J., Falkow S. Molecular nature of two nonconjugative plasmids carrying drug resistance genes. J Bacteriol. 1974 Feb;117(2):619–630. doi: 10.1128/jb.117.2.619-630.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyurasits E. B., Wake R. G. Bidirectional chromosome replication in Bacillus subtilis. J Mol Biol. 1973 Jan;73(1):55–63. doi: 10.1016/0022-2836(73)90158-7. [DOI] [PubMed] [Google Scholar]
- Heffron F., Rubens C., Falkow S. Translocation of a plasmid DNA sequence which mediates ampicillin resistance: molecular nature and specificity of insertion. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3623–3627. doi: 10.1073/pnas.72.9.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., Sublett R., Hedges R. W., Jacob A., Falkow S. Origin of the TEM-beta-lactamase gene found on plasmids. J Bacteriol. 1975 Apr;122(1):250–256. doi: 10.1128/jb.122.1.250-256.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R., Mayer A., Levine A. Replicating SV40 molecules containing closed circular template DNA strands. Nat New Biol. 1971 Sep 15;233(37):72–75. doi: 10.1038/newbio233072a0. [DOI] [PubMed] [Google Scholar]
- Lovett M. A., Sparks R. B., Helinski D. R. Bidirectional replication of plasmid R6K DNA in Escherichia coli; correspondence between origin of replication and position of single-strand break in relaxed complex. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2905–2909. doi: 10.1073/pnas.72.8.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters M., Broda P. Evidence for the bidirectional replications of the Escherichia coli chromosome. Nat New Biol. 1971 Aug 4;232(31):137–140. doi: 10.1038/newbio232137a0. [DOI] [PubMed] [Google Scholar]
- Nishioka Y., Eisenstark A. Sequence of genes replicated in Salmonella typhimurium as examined by transduction techniques. J Bacteriol. 1970 May;102(2):320–333. doi: 10.1128/jb.102.2.320-333.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman A., Champoux J. J., Dulbecco R. Characterization of the replicative intermediates of polyoma virus. Virology. 1974 Jan;57(1):147–160. doi: 10.1016/0042-6822(74)90116-0. [DOI] [PubMed] [Google Scholar]
- Rubens C., Heffron F., Falkow S. Transposition of a plasmid deoxyribonucleic acid sequence that mediates ampicillin resistance: independence from host rec functions and orientation of insertion. J Bacteriol. 1976 Oct;128(1):425–434. doi: 10.1128/jb.128.1.425-434.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnös M., Inman R. B. Position of branch points in replicating lambda DNA. J Mol Biol. 1970 Jul 14;51(1):61–73. doi: 10.1016/0022-2836(70)90270-6. [DOI] [PubMed] [Google Scholar]
- Sebring E. D., Kelly T. J., Jr, Thoren M. M., Salzman N. P. Structure of replicating simian virus 40 deoxyribonucleic acid molecules. J Virol. 1971 Oct;8(4):478–490. doi: 10.1128/jvi.8.4.478-490.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. R., Humphreys G. O., Anderson E. S. Genetic and molecular characterisation of some non-transferring plasmids. Mol Gen Genet. 1974 Mar 27;129(3):229–242. doi: 10.1007/BF00267915. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Weisblum B. Construction of a colicin E1-R factor composite plasmid in vitro: means for amplification of deoxyribonucleic acid. J Bacteriol. 1975 Jan;121(1):354–362. doi: 10.1128/jb.121.1.354-362.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmis K., Cabello F., Cohen S. N. Covalently closed circular DNA molecules of low superhelix density as intermediate forms in plasmid replication. Nature. 1976 Jun 10;261(5560):512–516. doi: 10.1038/261512a0. [DOI] [PubMed] [Google Scholar]




