Abstract
In vitro studies suggest an important role for CEACAM1 (carcinoembryonic antigen-related cell adhesion molecule 1) in infection by multiple gram-negative bacteria. However, in vivo evidence supporting this role is lacking, largely because the bacterial adhesins involved in this host-microbe association do not bind to murine-derived CEACAM1. One of several adhesins expressed by nontypeable Haemophilus influenzae (NTHI), the outer membrane protein P5-homologous adhesin (or P5), is essential for colonization of the chinchilla nasopharynx and infection of the middle ear. Here we reveal that NTHI P5 binds to the chinchilla homologue of CEACAM1 and that rabbit anti-human carcinoembryonic antigen blocks NTHI colonization of the chinchilla nasopharynx, providing the first demonstration of a role for CEACAM receptor binding by any bacterial pathogen in vivo.
The carcinoembryonic antigen-related cellular adhesion molecules (CEACAMs) are a subset of the immunoglobulin superfamily that mediate intercellular binding by homophilic and heterophilic interactions which influence a variety of normal and pathogenic processes, cellular growth, differentiation, and activation (reviewed in references 13, 16, 25, and 35). In vitro studies from several laboratories have now clearly established that adhesins expressed by a variety of important human-restricted bacterial pathogens bind specific members of the CEACAM family. For example, the evolutionarily unrelated colony opacity-associated (Opa) family of adhesins expressed by pathogenic and commensal Neisseria spp. (8, 10-12, 14, 15, 51-53), the Haemophilus influenzae outer membrane protein (OMP) P5 (20), and the Moraxella catarrhalis ubiquitous surface protein UspA1 (21) each bind CEACAMs. This interaction appears to mediate both attachment and engulfment of the bound bacteria by CEACAM-expressing epithelia (52, 54), endothelia (33, 34), and phagocytes (10, 14, 29, 46, 47, 52). In the context of the epithelia, polarized monolayers of epithelia express CEACAM1, CEACAM5, and CEACAM6 on the apical surface and at cell-cell junctions (49, 54). Bacterial binding to these receptors is sufficient to mediate apical adherence and transcellular transcytosis across the monolayers, allowing the bacteria to emerge in the subepithelial spaces (54). Bacterial binding to CEACAM1 also causes the direct suppression of immune cell activity due to the coinhibitory function of CEACAM1 (9). While these studies suggest an essential role for CEACAM binding in host colonization and persistence, the exquisite specificity of the protein-protein interactions mediated by each of these adhesins has so far prevented their contribution to infection from being addressed in vivo.
We previously demonstrated that nontypeable H. influenzae (NTHI) effectively colonizes the upper respiratory tract (URT) of chinchillas and that when this rodent host is also coinfected with a common human URT virus, NTHI can induce culture-positive otitis media (or middle ear infection) via a process that closely reflects the disease course in human children (5, 24, 38). In this viral-bacterial superinfection model system, intranasal administration of bacteria via passive inhalation leads to the establishment of colonization of the nasopharynx (NP), followed by ascension of the virus-compromised Eustachian tube and invasion of the middle ear space (3, 31, 32, 50). This process requires the NTHI P5 protein (also known as OMP P5-homologous adhesin, fimbriae, and OMP P5), as isogenic strains lacking this adhesin are significantly compromised in the ability to both colonize the NP and establish an infection in the middle ear (48). Moreover, P5-specific antisera are protective against experimental otitis media (5, 6, 24, 26). These observations prompted us to determine if the NTHI P5 protein binds chinchilla-derived CEACAMs and to ascertain whether this association contributes to pathogenesis in this important rodent model of human disease.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
NTHI strains 86-028NP and 1128 are low-passage clinical isolates recovered from children undergoing tympanostomy and tube insertion for chronic otitis media. Both have been described previously (18, 48). Strain 1128f− contains a chloramphenicol resistance cassette within the omp5 gene and therefore does not express the OMP P5-homologous adhesin (48). Strains 86-028NP/pKMLN-01 and 1128/pKMLN-01 both contain a reporter plasmid in which expression of the lux operon is under the control of the strong OMP P2 promoter (27). NTHI strain 86-028NP/pRSM2211 was made in the same genetic background as the Lux reporter construct, but this strain contains a plasmid in which expression of green fluorescent protein is under the control of the OMP P2 promoter (28). All NTHI isolates used here were grown at 37°C for 18 h on chocolate agar (Fisher Scientific, Pittsburgh, PA) in a humidified atmosphere of 5% CO2 and 95% air prior to use for in vitro or in vivo assays.
Fluorescence-activated cell sorter (FACS) analysis to determine specificity of binding of the selected NTHI clinical isolates to human CEACAM1.
HeLa cells that had been transfected to express human CEACAM1 (HeLa-CCM1 cells) or that had been transfected with the empty vector (HeLa-Neo cells) (14) were grown to 80% confluence and trypsinized (1 ml trypsin was added to the flask, followed by incubation for 1 min at 37°C), 2 ml medium was added to the flask, and cells were recovered by centrifugation at 1,200 rpm at 25°C for 5 min. Cells were then resuspended in fresh medium, counted, and adjusted to 1e6 viable cells/ml. Five hundred microliters of this cell suspension was transferred to a sterile Eppendorf tube, and cells were pelleted (centrifugation at 1,400 rpm at 25°C for 5 min), resuspended in 100 μl 1× Dulbecco's phosphate-buffered saline (DPBS) (pH 7.4) containing 0.05% bovine serum albumin (Fisher Scientific, Pittsburgh, PA), and allowed to incubate at room temperature for 15 min. Polyclonal rabbit anti-human carcinoembryonic antigen (CEA) antibody (dialyzed to remove azide and then diluted 1:10) (Dako, Carpinteria, CA) was then added to samples at the concentration indicated and allowed to incubate at room temperature for 45 min. This antibody reacts with both CEA (CEACAM5) and CEACAM1. Following incubation, cells were washed three times with 1× DPBS and then resuspended in 250 μl of the same buffer.
To prepare bacteria for use in the FACS-based assay, NTHI strain 86-028NP/pRSM2211 was grown on chocolate agar for 18 h, and colonies were collected and suspended in 1× DPBS to an optical density at 490 nm of 0.6. Five hundred microliters of this bacterial suspension was added to each epithelial cell sample and allowed to incubate at 37°C with 5% CO2 for 2 h. Following incubation, cells were washed three times with 1× DPBS to remove nonadherent bacteria, suspended in 400 μl 1× DPBS, and analyzed by flow cytometry.
For detection of either NTHI strain 1128 or its isogenic P5 mutant (1128f−), bacteria were first labeled with 1% biotin by incubation on ice for 45 min, as previously described (39, 48), prior to adding biotin-labeled bacteria to epithelial cells. After incubation and washes as described above, biotinylated NTHI cells that were adherent to epithelial cells were detected using phycoerythrin-streptavidin (Zymed, San Francisco, CA). We have previously shown that biotinylation does not interfere with adherence of NTHI to respiratory epithelial cells (4). All adherence assays were repeated a minimum of three times, on separate days. Data are reported as mean values ± standard deviations.
Biacore analysis of interaction between soluble recombinant human CEACAM1 and synthetic peptides designed to mimic the four predicted surface-exposed regions of the NTHI OMP P5-homologous adhesin.
Synthetic peptides (each 24 to 28 residues in length) designed to mimic the four predicted surface-exposed regions of the OMP P5-homologous adhesin (called region 1 to region 4 peptides) were bound to a Biacore chip as previously described (39, 41). Soluble recombinant CEACAM1 (srCEACAM1) isolated from transfected cells was dialyzed and concentrated, also as described previously (56). Briefly, the cell supernatant was dialyzed against water overnight at 4°C, using a 3.5-kDa-molecular-size-cutoff dialysis cassette (Novagen, Madison, WI). The sample was placed in an UltraFree-4 centrifugal filter unit containing a 5K NMWL membrane (Millipore, Bedford, MA) and centrifuged in an Eppendorf 5810R tabletop centrifuge (Eppendorf AG, Hamburg, Germany) at 4,000 rpm until concentrated. The concentrated srCEACAM1 was then diluted 1:2 in buffer containing 1 mg carboxymethyl dextran/ml (55), and a 15-μl sample was assayed at a flow rate of 10 μl/min across each of the four flow cells, to which one of the four region peptides had been bound. The interaction between these proteins was evaluated in real time and reported in resonance units, as we have described previously (39, 41).
Immunohistochemical evaluation of chinchilla URT mucosa to detect expression of a human CEA homologue.
Chinchilla middle ear epithelial cells (CMEE cells) were established as previously described (36, 37), and 2e5 cells were seeded into a transwell insert (6.5 mm; Costar, Corning, NY). Cells were allowed to grow to confluence before medium was removed from the top chamber only, thereby allowing further culture of these cells at the air-fluid interface. While polarizing, cells were fed with CMEE growth medium (36, 37) via the lower chamber of the transwell every 2 days for 1 week. Following polarization, the transwell membrane on which the CMEE cells had grown was embedded in paraffin and sectioned into 4-μm sections for immunolabeling. Slides were labeled using a 1:500 dilution of murine monoclonal anti-human CD66a antibody (NovoCastra, Newcastle, United Kingdom) or mouse immunoglobulin G (IgG; Dako, Carpinteria, CA) as an isotype control, followed by incubation with a 1:200 dilution of horseradish peroxidase (HRP)-conjugated goat anti-mouse antiserum (Zymed, Carlsbad, CA), using diaminobenzidine (DAB) as the chromogen (Biocare Medical, Walnut Creek, CA), according to the manufacturer's instructions. Sections were viewed and analyzed using a Zeiss Axioskop 40 light microscope (Carl Zeiss Inc., Thornwood, NY).
Whole chinchilla Eustachian tubes were recovered from a naïve animal, embedded in OCT fixative, and sectioned using methods described previously (23). Sections were labeled using a 1:500 dilution of fluorescein isothiocyanate (FITC)-conjugated murine monoclonal anti-human CD66abce antibody (Kat4c clone; Dako, Denmark) or FITC-conjugated mouse IgG (Dako) as an isotype control. Biotin-labeled phalloidin (Sigma, St. Louis, MO), detected with streptavidin-conjugated phycoerythrin, was used as a cytoskeletal counterstain, and sections were analyzed using a laser scanning confocal microscope (Zeiss 510 Meta).
Western blot analysis of whole-cell lysates to demonstrate relative expression of CEA among epithelial cells used in these studies.
Epithelial cells (CMEE, HeLa-CCM1, HeLa-Neo, and CHO cells) were seeded and grown to 80% confluence. EDTA (1.8% [wt/vol] in Tris-buffered saline) was then added to flasks to dissociate cells. Cells were counted, and aliquots containing equal concentrations of cells were pelleted, resuspended in 100 μl Tris-buffered saline, solubilized by being mixed with solubilizing buffer containing β-mercaptoethanol and sodium dodecyl sulfate (SDS), boiled, and loaded into lanes of a 12% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) gel. After electrophoresis (150 V, 35 min), separated proteins were transferred to 45-μm-pore-size nitrocellulose and labeled with a 1:1,000 dilution of polyclonal rabbit anti-human CEA antibody, followed by incubation with a 1:5,000 dilution of HRP-conjugated goat anti-rabbit antibody and detection with CN/DAB substrate (Pierce, Rockford, IL). In preparation for subsequent in vivo studies, CMEE cell lysates were also incubated with rabbit anti-human E-cadherin (NeoMarkers, Fremont, CA) (diluted 1:2,000), followed by incubation with HRP-conjugated goat anti-rabbit antibody (diluted 1:2,000) and detection with CN/DAB as described above.
Expression profiling by RT-PCR.
Chinchilla tissues were collected from a naïve animal and stored at −80°C. Total RNA was isolated from the samples, using previously described methods (30), and the integrity of the purified RNA was evaluated using an Agilent 2100 bioanalyzer (Agilent, Foster City, CA). For reverse transcription-PCR (RT-PCR), chinchilla CEACAM1-F (5′-CCCCCAGACTCCTACTTCCATC-3′) and chinchilla CEACAM1-R (5′-ATACTCCCCGGCATCCTGTC-3′) primers with 10 nanograms of total RNA were used in 25-μl amplification reaction mixtures, using a QuantiTect SYBR green RT-PCR system (Qiagen, Valencia, CA). The sequences of the primers were based on a partial chinchilla CEACAM1 (cCEACAM1) cDNA generated from URT tissue RNA (data not shown). Reaction conditions for the one-step RT-PCR procedure were 30 min of RT at 50°C, followed by heating to 95°C for 15 min. A 35-cycle three-step procedure was then used, which consisted of repeated denaturation at 94°C for 15 seconds, annealing at 55°C for 30 seconds, and then extension at 72°C for 30 seconds. Amplicons generated from reactions with or without reverse transcriptase, to confirm the absence of contaminating genomic DNA, were separated in a 0.8% agarose gel and stained with ethidium bromide.
Determining the effect of blocking CEA on the ability of NTHI to colonize the chinchilla NP in vivo.
Polyclonal rabbit anti-human CEA (Dako, Denmark) or rabbit Ig (Dako, Denmark) was diluted 1:2 in sterile pyrogen-free saline (Hospira Inc., Lake Forest, IL) prior to delivery to chinchillas. Before we conducted this study, we determined that neither rabbit anti-human CEA nor rabbit Ig diminished luminescence by NTHI strain 86-028NP/pKMLN-01 by spreading an undiluted aliquot of each of these sera onto chocolate agar prior to plating the luminescent microbe and comparing the luminescence on each plate to the luminescence obtained when this reporter was plated on chocolate agar on which no rabbit serum had been spread. All cultures yielded equivalent numbers of colonies, and all colonies luminesced as brightly as those obtained by spreading cells onto plain chocolate agar (data not shown).
Six adult chinchillas (Chinchilla lanigera) were first lightly anesthetized using ketamine and xylazine prior to intranasal administration of 200 μl diluted anti-CEA antibody or naïve rabbit Ig by passive inhalation, with the dosage divided equally between the nares. Chinchillas were then allowed to rest in a prone position for 20 min prior to intranasal challenge with 200 μl of a suspension of NTHI strain 86-028NP/pKMLN-01 (colonies were recovered after 18 to 24 h of growth on chocolate agar and suspended to an optical density at 490 nm of 0.65 in sterile pyrogen-free saline) by passive inhalation as described previously (5, 24, 38). NP lavage was performed, also as previously described (5, 24, 38), on days 1, 4, and 7 after bacterial challenge, and lavage fluids were diluted and plated to enumerate recovered NTHI cells/ml NP lavage fluid. Challenged animals, recovered NP lavage fluids, and colonies resulting from dilution plating of NP lavage fluids were also imaged for the presence of luminescent bacteria (27, 40). Kaplan-Meier curves of time to clearance were compared by the Wilcoxon test. P values of <0.05 were considered significant.
In our second study, two adult chinchillas were administered either rabbit anti-human CEA or rabbit anti-human E-cadherin (NeoMarkers, Fremont, CA) 20 minutes before intranasal challenge with NTHI strain 1128/pKMLN-01 as described above. NP lavage was performed 24 hours after bacterial challenge, and lavage fluids were subjected to biophotonic imaging, also as already described.
RESULTS
Demonstration that NTHI strain 86-028NP uses human CEACAM1 as a receptor in vitro.
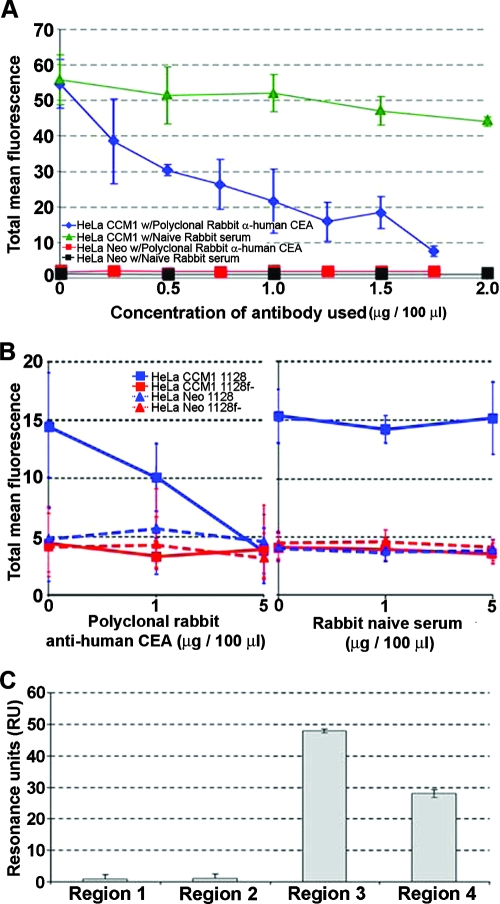
In order to first verify that NTHI strain 86-028NP binds to CEACAM1, as already shown for other NTHI isolates (20), we attempted to block adherence of the green fluorescent protein reporter construct-expressing strain (86-028NP/pRSM2211) to HeLa cells transfected to express extracellular CEACAM1 (HeLa-CCM1) by using a rabbit anti-human CEA antibody which cross-reacts with human CEACAM1. As determined by FACS analysis, Fig. 1A shows that the ability of strain 86-028NP/pRSM2211 to bind to HeLa-CCM1 cells was effectively blocked, in a dose-dependent manner, by preincubation of the epithelial target cells with increasing concentrations of CEACAM-specific antibody (blue diamond plot). Conversely, adherence of this NTHI isolate to HeLa-CCM1 cells was essentially uninhibited by preincubation of these epithelial cells with similar concentrations of purified rabbit Ig serum (green triangle plot). As an additional control, HeLa cells transfected with the empty vector (HeLa-Neo) did not support adherence of NTHI strain 86-028NP/pRSM2211, regardless of whether the target cells were preincubated with either CEACAM-specific antibody or the control rabbit Ig serum (red and black square plots, respectively).
FIG. 1.
(A) Relative adherence of NTHI strain 86-028NP to HeLa cells transfected to express human CEACAM1 (HeLa-CCM1) or to HeLa cells containing empty vector (HeLa-Neo) when epithelial cells were preincubated with increasing concentrations of either naïve rabbit serum or polyclonal rabbit anti-human CEA antibody. (B) Relative adherence of an NTHI isolate that expresses the P5 adhesin (strain 1128) or an isogenic mutant that does not (strain 1128f−) to HeLa-Neo or CCM1 cells after preincubation of the epithelial cells with rabbit anti-human CEA (left) or naïve rabbit serum (right). (C) Relative binding of soluble recombinant human CEACAM1 to synthetic peptides designed after the four predicted surface-exposed regions of the NTHI P5 adhesin, as measured by biosensor. Data are reported in mean resonance units (RU) ± standard deviations.
Demonstration that NTHI binding to human CEACAM1 is mediated by the OMP P5-homologous adhesin.
To demonstrate that the adherence of NTHI to HeLa cells that expressed rCEACAM1 was dependent on expression of the OMP P5-homologous adhesin, biotin-labeled wild-type NTHI or its isogenic P5 mutant was incubated with either HeLa-CCM1 or HeLa-Neo cells, and adherent NTHI cells were detected via FACS analysis. Figure 1B shows a dose-dependent inhibition of adherence of NTHI to HeLa-CCM1 when these cells were preincubated with increasing concentrations of polyclonal rabbit anti-human CEA antibody (left panel), an effect that was not seen when naïve rabbit serum was used (right panel). As shown previously (Fig. 1A), NTHI adhered to HeLa-Neo cells at a notably reduced level. The isogenic P5 mutant also showed a limited ability to adhere to HeLa-CCM1 cells, consistent with the relative importance of the P5 adhesin for binding of NTHI via CEACAM1. This limited adherence of the P5 mutant was not mitigated further by preincubation of HeLa-Neo cells with either CEACAM-specific or naïve rabbit serum.
NTHI bound to srCEACAM1 via the third and fourth predicted surface-exposed loops of the OMP P5-homologous adhesin.
While it has been shown that NTHI binds CEACAM1 via the OMP P5-homologous adhesin, there have been no data yet presented that attempt to define which domain of this bacterial surface protein mediates interaction with CEACAM1. Figure 1C depicts data obtained when isolated srCEACAM1 was allowed to interact with synthetic peptides representing the four predicted surface-exposed regions of P5 that had been bound to a Biacore chip and real-time protein-protein interactions were measured as isolated srCEACAM1 was allowed to flow across the chip surface. The results demonstrate that peptides representing surface-exposed regions 3 and 4 bound the greatest amounts of srCEACAM1, whereas those representing regions 1 and 2 showed minimal interaction with this eukaryotic cell adhesion molecule. In contrast, soluble CEACAM5 antigen (Fitzgerald, Concord, MA) did not bind to any of the four region peptides (data not shown). These data support those previously published by our group whereby regions 3 and 4 were defined as adhesin-binding domains of the OMP P5-homologous adhesin, assayed with human oropharyngeal cells (39).
Demonstration that a CEACAM1 homologue is expressed on the apical surface of chinchilla respiratory tract epithelia.
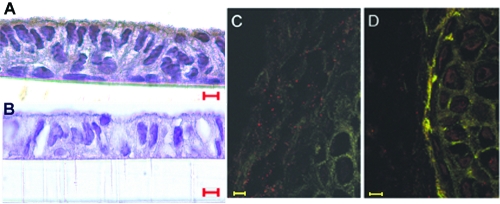
Before attempting to demonstrate the involvement of a chinchilla CEACAM1 homologue-NTHI P5 interaction in vivo by using a rodent model system, we first wanted to determine if the chinchilla host expressed a homologue of human CEACAM1 on its epithelial cell surface. Figure 2 shows strong labeling of polarized CMEE cells incubated with murine monoclonal anti-human CEACAM1 antibody (Fig. 2A) but not of those incubated with a murine monoclonal IgG control (Fig. 2B), as detected using DAB as the chromogen. In Fig. 2C and D, we demonstrate the absence of labeling of a cross section of a whole chinchilla Eustachian tube when the isotype control serum (FITC-conjugated mouse IgG) was used (Fig. 2C) but positive labeling of the Eustachian tube mucosal surface when FITC-conjugated murine monoclonal anti-human CD66abce was used (Fig. 2D).
FIG. 2.
Demonstration of expression of a human CEACAM1 homologue by chinchilla respiratory tract epithelial cells, using immunohistochemistry (A and B) and fluorescence microscopy (C and D). Polarized CMEE cells are depicted in panels A and B, wherein positive labeling for CEACAM1 is depicted by dark brown staining. Serial cross sections of whole frozen chinchilla Eustachian tubes are shown in panels C and D, wherein positive labeling is shown by bright fluorescence at the mucosal surface of the Eustachian tube section shown in panel D. For panels B and C, mouse IgG and FITC-conjugated mouse IgG were used as isotype controls for the similarly unconjugated and FITC-conjugated murine monoclonal anti-human CEACAM1-specific antisera used as the primary antibodies in panels A and D, respectively. Bars, 5 μm (A and B) and 20 μm (C and D).
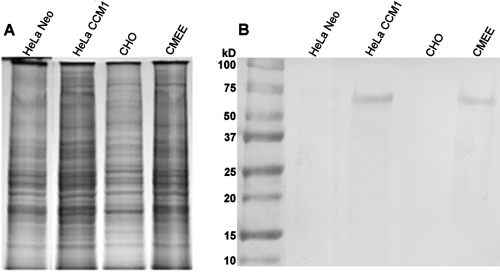
In support of the data obtained with cultured CMEE cells and whole frozen chinchilla Eustachian tubes, Fig. 3 shows a Western blot obtained after separation of epithelial whole-cell lysates by SDS-PAGE, followed by incubation with polyclonal rabbit anti-human CEA (Fig. 3B). Whole-cell lysates of HeLa-CEACAM1 cells showed similar labeling of an approximately 150-kDa band to that obtained using a lysate of CMEE cells, while cells transfected with empty vector (HeLa-Neo) or CHO cells demonstrated no labeling. Collectively, our data suggested that the chinchilla host expressed a homologue of human CEACAM1 in the middle ear and Eustachian tube, both of which are primary sites for the presence of NTHI during the disease course of otitis media.
FIG. 3.
Whole-cell lysates of HeLa-Neo, HeLa-CCM1, CHO, and CMEE cells, separated by SDS-PAGE (A) and shown after Western blotting using a rabbit anti-human CEA antibody (B). Note the presence of an ∼150-kDa band in lanes containing lysates of HeLa-CCM1 or CMEE cells.
CMEE cell lysates also bound rabbit anti-human E-cadherin antibody, with a band hybridizing at approximately 98 kDa, as expected (data not shown). This band was not present when the HRP-conjugated secondary antibody was used in the absence of rabbit anti-human E-cadherin. This finding was used in support of subsequent in vivo studies described below.
Demonstration of sites of constitutive expression of the chinchilla CEACAM1 homologue by expression profiling.
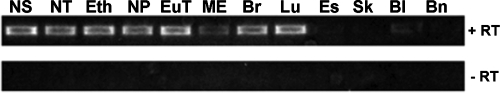
As a step towards more specifically defining the expression of cCEACAM1 within individual tissue sites, we isolated total RNAs from samples and used RT-PCR to amplify cCEACAM1 transcripts (Fig. 4). For a naïve chinchilla, cCEACAM1 mRNA was detected in each URT tissue evaluated, including mucosae of the nasal septum, nasoturbinate, ethmoid turbinate, NP, Eustachian tube, and middle ear. In addition, cCEACAM1 mRNA was also detected in the lower airway, including the bronchus and lung. A weak signal was obtained using mRNAs recovered from bladder mucosa, but none was detected when samples from either the brain, skin, or esophagus were assayed. These expression data were consistent with what is known regarding CEACAM1 expression in humans (44), rats (42, 43), and mice (17) and, furthermore, demonstrated constitutive expression of cCEACAM1 throughout the upper and lower respiratory tracts.
FIG. 4.
RT-PCR analysis of cCEACAM1 transcripts in chinchilla tissues. Amplicons generated with (+RT) or without (−RT) reverse transcriptase are shown. cCEACAM1 transcripts were detected in the nasal septum (NS), nasoturbinate (NT), ethmoid turbinate (Eth), NP, Eustachian tube (EuT), middle ear mucosa (ME), bronchus (Br), and lung (Lu) but not in the esophagus (Es), skin (Sk), or brain (Bn) and were only weakly detected in the bladder mucosa (Bl).
Demonstration of NTHI P5 adhesin-chinchilla CEACAM1 homologue interaction in vivo.
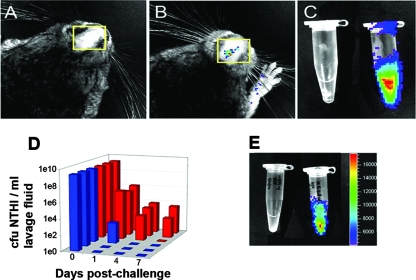
We know that P5 is absolutely required for experimental otitis media because a P5-deficient strain is significantly impaired in the ability to colonize the chinchilla NP and to infect the middle ear (48). To determine whether the chinchilla CEACAM1 homologue plays a role in colonization of the chinchilla NP by NTHI, we measured relative bacterial concentrations present in NP lavage fluids over time after animals were given either rabbit anti-human CEA antibody or nonimmune rabbit Ig 20 minutes prior to challenge with a luminescent NTHI reporter strain. Figure 5 depicts biophotonic images of a representative chinchilla that was given anti-human CEA antibody (Fig. 5A) and a chinchilla that received nonimmune rabbit Ig (Fig. 5B). These images were captured 19 h after challenge of the nasal cavities of both animals with the luminescent NTHI strain. Whereas luminescent bacteria were not detected in the chinchilla given the anti-CEA antibody, we were able to detect luminescent NTHI cells in the NP of the chinchilla that received nonimmune rabbit serum. NP lavage fluids recovered from an additional animal in each of these cohorts 1 day (24 h) after bacterial challenge were also subjected to biophotonic imaging (Fig. 5C), demonstrating a dramatic reduction in the amount of bacteria recovered in the presence of CEACAM-specific antibody (see the Eppendorf tube on the left) versus that obtained from the NP of a chinchilla that had received nonimmune rabbit serum (see the Eppendorf tube on the right).
FIG. 5.
(A) Biophotonic image of a chinchilla from the cohort that received rabbit anti-human CEA antibody 20 min prior to challenge with a luminescent NTHI reporter strain. This image was captured 19 h after bacterial challenge and shows no detection of luminescence from the nasal cavity (yellow boxed region). A similar image from an animal that received naïve rabbit serum is shown in panel B and demonstrates the detection of luminescence from the nasal cavity of this chinchilla (see the yellow boxed region). (C) Biophotonic image of NP lavage fluids recovered 24 h after bacterial challenge from animals in the cohorts that received either rabbit anti-human CEA (left tube) or naïve rabbit Ig (right tube). (D) Relative clearance of NTHI from the NP of chinchillas in cohorts that received either naïve rabbit serum (red bars) or rabbit anti-human CEA (blue bars) prior to intranasal bacterial challenge. Chinchillas that received anti-human CEA antibody cleared NTHI from their NP significantly earlier than did those that received naïve rabbit serum (P = 0.031). (E) Biophotonic image of NP lavage fluids recovered 24 h after bacterial challenge from animals that received either rabbit anti-human CEA antiserum (tube on left) or rabbit anti-human E-cadherin antiserum (tube on right).
Figure 5D depicts the kinetics of clearance of the Lux-expressing NTHI strain 86-028NP from the NP of each chinchilla in both of the described cohorts. Two of the three animals within the cohort administered CEACAM-specific antibody (blue bars) were cleared of culturable NTHI within the first 24 h of challenge, with the third chinchilla eradicating NTHI from its NP within a maximum period of 4 days after challenge. However, 24 h after challenge, the bacterial load in the NP of the latter animal was already reduced nearly 7 log relative to the challenge inoculum (3.8e2 compared to 2.2e9 CFU/ml). Conversely, this strain of NTHI was able to maintain colonization of the NP of two of the three animals administered nonimmune rabbit Ig for at least 7 days (red bars), which was the maximum period of observation in the study. The third animal in this cohort was colonized for a minimum of 4 days after challenge. Thus, the estimated mean clearance time for the cohort administered anti-CEA was 2 days, whereas that for the group given nonimmune rabbit IgG was 7 days. The median clearance time for the groups could not be computed due to the small sample sizes and the censored data in one of the groups (i.e., two animals in the cohort that received nonimmune rabbit Ig had not yet cleared NTHI from their NP at the time the study was ended). The Kaplan-Meier curves for time to clearance were significantly different (P = 0.031). Collectively, our data suggested that the chinchilla CEACAM1 homologue plays a pivotal role in NTHI colonization of the chinchilla NP.
To demonstrate that the clearance of NTHI from the chinchilla NP was indeed due to the action of antibody-mediated blockade of a receptor used for bacterial adherence, as well as to show that the observed outcome was not NTHI strain specific, we repeated the experiment described above, using another clinical isolate as well as an additional antiserum. Two adult chinchillas were given either rabbit anti-human CEA or rabbit anti-human E-cadherin serum 20 minutes prior to challenge with Lux-expressing NTHI strain 1128. Twenty-four hours after NTHI challenge, we obtained NP lavage fluids from these animals and subjected them to biophotonic imaging, as shown in Fig. 5E. The Eppendorf tube on the left contains NP lavage fluid recovered from a chinchilla given anti-human CEA serum, whereas the tube on the right contains NP lavage fluid recovered from a chinchilla administered anti-human E-cadherin serum. Again, anti-CEA, but not anti-E-cadherin, serum resulted in inhibition of colonization by a second NTHI isolate, strain 1128, suggesting that CEACAM1 was indeed essential for NTHI colonization of the chinchilla NP, whereas no effect was apparent with antiserum directed towards a closely related molecule (e.g., E-cadherin) to which NTHI does not bind.
DISCUSSION
We previously established that the OMP P5-homologous adhesin (2, 6, 7, 22, 32, 45) is a virulence determinant that is required for NP colonization and the induction of experimental otitis media by NTHI in chinchillas (48). Due to these observations, we have developed P5-derived vaccine candidates that have proven efficacy in mediating clearance of NTHI from the NP as well as protection from induction and a reduction in severity of disease in experimental models of NTHI-induced otitis media (5, 6, 24, 26). However, while the role of P5 in colonization of the chinchilla NP and pathogenesis of otitis media has been well documented, its function in vivo has not been defined previously.
Herein, we have confirmed that the well-characterized NTHI strain 86-028NP binds to human CEACAM1 expressed by transfected HeLa cells in vitro. This interaction could be blocked with rabbit antiserum directed against CEACAM family receptors but not by naïve rabbit serum. Consistent with a role for the bacterial P5 protein in this interaction, an isogenic mutant lacking the P5 adhesin (6, 39, 48) lost its ability to adhere to HeLa cells in a CEACAM-dependent manner. This binding appeared to be mediated via surface-exposed regions 3 and 4 of the OMP P5 adhesin, as peptide mimics of these regions mediated binding to soluble CEACAM1 in a biosensor analysis.
Due to the observed efficacy of P5-based immunogens in chinchilla models of bacterial otitis media, we were interested in the molecular mechanisms that underlie the essential nature of P5 during infection. We initially sought to determine whether a homologue of human CEACAM1 was available in the chinchilla URT and other tissues to serve as a receptor for P5-mediated adherence. Using human-specific reagents, we utilized both immunohistochemistry and fluorescence confocal microscopy to observe the expression of a CEA homologue on both the apical surface of primary epithelial cells grown from the chinchilla middle ear and the mucosal surface of cells lining the chinchilla Eustachian tube. Furthermore, by Western blot analysis, we were able to show, using CMEE whole-cell lysates, that CMEE cells constitutively expressed a human CEA homologue, at a level that approximated that of HeLa cells that had been transfected to do so. Using expression profiling analysis by RT-PCR, we showed that cCEACAM1 was expressed throughout the chinchilla airway. When we evaluated tissues from multiple sites in the chinchilla host, we found that all tissues recovered from the respiratory tract (i.e., nasal septum, nasoturbinate, ethmoid turbinate, NP, Eustachian tube, middle ear, bronchus, and lung) yielded a positive signal, suggesting that this receptor is available for use in bacterial colonization. We could not detect the CEACAM1 homologue in esophagus, skin, or brain tissue samples, which were negative by these methods.
After establishing that the chinchilla host expressed a human CEACAM1 homologue, we sought to confirm that this protein functioned as a receptor for NTHI in vivo. Considering our observation that anti-CEACAM serum effectively blocked NTHI binding to CEACAM1-expressing cell lines in vitro, we attempted to block NTHI adherence to and colonization of the chinchilla NP by administering antibody directed against human CEA to the nasal cavity mucosa prior to intranasal challenge with a low-passage NTHI clinical isolate. Whereas we had anticipated that this antibody might ameliorate adherence of this bacterium, we instead saw that the CEACAM-specific antibodies completely abrogated NP colonization by two different NTHI isolates. In striking contrast, chinchillas that were given rabbit Ig remained colonized with NTHI strain 86-028NP/pKMLN-01 for at least 7 days postchallenge. Moreover, the mean time to clearance for the animals that received rabbit anti-human CEA antibody was 2 days, a 5-day difference from that for control animals, which was statistically significant. To ensure that this effect was not restricted to a particular NTHI strain but was specific to a known NTHI receptor, we conducted a second small study in which we administered antiserum against either human CEA or human E-cadherin, a CEACAM1-related protein to which NTHI does not adhere. Whereas rabbit anti-human CEA effectively inhibited our ability to recover Lux-expressing NTHI strain 1128 from the NP of a chinchilla 24 h after challenge, the presence of anti-human E-cadherin did not affect NTHI colonization of the chinchilla NP.
Collectively, our data support several conclusions. Firstly, the chinchilla host, via its expression of a human CEACAM1 homologue, can serve as a valuable model for the study of the pathogenic mechanisms utilized by several human-restricted mucosal pathogens that adhere to this member of the CEACAM family via a variety of adhesins. Secondly, these data provide an enhanced understanding of the observed efficacy of P5-derived vaccine candidates in the chinchilla host, wherein antibodies to P5 (or targeted surface-exposed regions thereof), via either active or passive immunization, have resulted in rapid clearance of NTHI from the NP and significantly reduced both the incidence and severity of middle ear disease in immune cohorts relative to controls (5, 6, 24, 26). Our most recent findings are also in keeping with prior observations that P5-derived peptides inhibit binding of NTHI to both chinchilla tracheal epithelium and human oropharyngeal cells in vitro (6, 39). Similarly, Hill et al. (19) have shown that a recombinant polypeptide derived from UspA1 of M. catarrhalis (another bacterial protein ligand of CEACAM) blocks adherence of not only M. catarrhalis but also Neisseria meningitidis and H. influenzae.
Thirdly, and perhaps most importantly, while numerous bacteria have been shown to adhere to CEACAM receptors in vitro (8, 10-15, 20, 51-53), the data herein provide the first demonstration that blockade of the bacterial adhesin-host cell CEACAM interaction allows rapid clearance of the microbe from an environmental niche in the mammalian respiratory tract. This concept is particularly intriguing given the propensity of several URT viruses that are commonly associated with bacterial superinfections to upregulate the expression of CEACAM1, as well as other receptors (1, 2), and thereby facilitate bacterial adherence, including P5-mediated adherence by NTHI (2). Thus, targeting this bacterium-host interaction for blockade or interference could have important implications for the development and use of novel therapeutics as well as CEACAM adhesin-targeted vaccine candidates for the expanding repertoire of bacteria that bind to CEACAM family receptors on the human mucosa.
Acknowledgments
We thank Henry Wong for the provision of soluble recombinant human CEACAM1, Stephanie Hill and Laura Novotny for performing Biacore analyses, and Jennifer Neelans for manuscript and figure preparation.
This work was supported by grant R01-DC03915 to L.O.B. from NIDCD/NIH. S.D.G. is supported by the Canadian Institutes for Health Research grant 15499.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 15 October 2007.
REFERENCES
- 1.Avadhanula, V., C. A. Rodriguez, J. P. Devincenzo, Y. Wang, R. J. Webby, G. C. Ulett, and E. E. Adderson. 2006. Respiratory viruses augment the adhesion of bacterial pathogens to respiratory epithelium in a viral species- and cell type-dependent manner. J. Virol. 801629-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avadhanula, V., C. A. Rodriguez, G. C. Ulett, L. O. Bakaletz, and E. E. Adderson. 2006. Nontypeable Haemophilus influenzae adheres to intercellular adhesion molecule 1 (ICAM-1) on respiratory epithelial cells and upregulates ICAM-1 expression. Infect. Immun. 74830-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakaletz, L. O., R. L. Daniels, and D. J. Lim. 1993. Modeling adenovirus type 1-induced otitis media in the chinchilla: effect on ciliary activity and fluid transport function of Eustachian tube mucosal epithelium. J. Infect. Dis. 168865-872. [DOI] [PubMed] [Google Scholar]
- 4.Bakaletz, L. O., T. Hoepf, and D. J. Lim. 1991. Inhibition of adherence of NTHi to human oropharyngeal cells—and ELISA assay, abstr. 145. Abstr. 5th Int. Symp. Recent Adv. Otitis Media.
- 5.Bakaletz, L. O., B. J. Kennedy, L. A. Novotny, G. Dequesne, J. Cohen, and Y. Lobet. 1999. Protection against development of otitis media induced by nontypeable Haemophilus influenzae by both active and passive immunization in a chinchilla model of virus-bacterium superinfection. Infect. Immun. 672746-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bakaletz, L. O., E. R. Leake, J. M. Billy, and P. T. Kaumaya. 1997. Relative immunogenicity and efficacy of two synthetic chimeric peptides of fimbrin as vaccinogens against nasopharyngeal colonization by nontypeable Haemophilus influenzae in the chinchilla. Vaccine 15955-961. [DOI] [PubMed] [Google Scholar]
- 7.Bakaletz, L. O., B. M. Tallan, T. Hoepf, T. F. DeMaria, H. G. Birck, and D. J. Lim. 1988. Frequency of fimbriation of nontypable Haemophilus influenzae and its ability to adhere to chinchilla and human respiratory epithelium. Infect. Immun. 56331-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bos, M. P., F. Grunert, and R. J. Belland. 1997. Differential recognition of members of the carcinoembryonic antigen family by Opa variants of Neisseria gonorrhoeae. Infect. Immun. 652353-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boulton, I. C., and S. D. Gray-Owen. 2002. Neisserial binding to CEACAM1 arrests the activation and proliferation of CD4+ T lymphocytes. Nat. Immunol. 3229-236. [DOI] [PubMed] [Google Scholar]
- 10.Chen, T., and E. C. Gotschlich. 1996. CGM1a antigen of neutrophils, a receptor of gonococcal opacity proteins. Proc. Natl. Acad. Sci. USA 9314851-14856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, T., F. Grunert, A. Medina-Marino, and E. C. Gotschlich. 1997. Several carcinoembryonic antigens (CD66) serve as receptors for gonococcal opacity proteins. J. Exp. Med. 1851557-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray-Owen, S. D. 2003. Neisserial Opa proteins: impact on colonization, dissemination and immunity. Scand. J. Infect. Dis. 35614-618. [DOI] [PubMed] [Google Scholar]
- 13.Gray-Owen, S. D., and R. S. Blumberg. 2006. CEACAM1: contact-dependent control of immunity. Nat. Rev. Immunol. 6433-446. [DOI] [PubMed] [Google Scholar]
- 14.Gray-Owen, S. D., C. Dehio, A. Haude, F. Grunert, and T. F. Meyer. 1997. CD66 carcinoembryonic antigens mediate interactions between Opa-expressing Neisseria gonorrhoeae and human polymorphonuclear phagocytes. EMBO J. 163435-3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray-Owen, S. D., D. R. Lorenzen, A. Haude, T. F. Meyer, and C. Dehio. 1997. Differential Opa specificities for CD66 receptors influence tissue interactions and cellular response to Neisseria gonorrhoeae. Mol. Microbiol. 26971-980. [DOI] [PubMed] [Google Scholar]
- 16.Hammarstrom, S. 1999. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin. Cancer Biol. 967-81. [DOI] [PubMed] [Google Scholar]
- 17.Han, E., D. Phan, P. Lo, M. N. Poy, R. Behringer, S. M. Najjar, and S. H. Lin. 2001. Differences in tissue-specific and embryonic expression of mouse Ceacam1 and Ceacam2 genes. Biochem. J. 355417-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrison, A., D. W. Dyer, A. Gillaspy, W. C. Ray, R. Mungur, M. B. Carson, H. Zhong, J. Gipson, M. Gipson, L. S. Johnson, L. Lewis, L. O. Bakaletz, and R. S. Munson, Jr. 2005. Genomic sequence of an otitis media isolate of nontypeable Haemophilus influenzae: comparative study with H. influenzae serotype d, strain KW20. J. Bacteriol. 1874627-4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill, D. J., A. M. Edwards, H. A. Rowe, and M. Virji. 2005. Carcinoembryonic antigen-related cell adhesion molecule (CEACAM)-binding recombinant polypeptide confers protection against infection by respiratory and urogenital pathogens. Mol. Microbiol. 551515-1527. [DOI] [PubMed] [Google Scholar]
- 20.Hill, D. J., M. A. Toleman, D. J. Evans, S. Villullas, L. Van Alphen, and M. Virji. 2001. The variable P5 proteins of typeable and non-typeable Haemophilus influenzae target human CEACAM1. Mol. Microbiol. 39850-862. [DOI] [PubMed] [Google Scholar]
- 21.Hill, D. J., and M. Virji. 2003. A novel cell-binding mechanism of Moraxella catarrhalis ubiquitous surface protein UspA: specific targeting of the N-domain of carcinoembryonic antigen-related cell adhesion molecules by UspA1. Mol. Microbiol. 48117-129. [DOI] [PubMed] [Google Scholar]
- 22.Jiang, Z., N. Nagata, E. Molina, L. O. Bakaletz, H. Hawkins, and J. A. Patel. 1999. Fimbria-mediated enhanced attachment of nontypeable Haemophilus influenzae to respiratory syncytial virus-infected respiratory epithelial cells. Infect. Immun. 67187-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jurcisek, J. A., J. E. Durbin, D. F. Kusewitt, and L. O. Bakaletz. 2003. Anatomy of the nasal cavity in the chinchilla. Cells Tissues Organs 174136-152. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy, B. J., L. A. Novotny, J. A. Jurcisek, Y. Lobet, and L. O. Bakaletz. 2000. Passive transfer of antiserum specific for immunogens derived from a nontypeable Haemophilus influenzae adhesin and lipoprotein D prevents otitis media after heterologous challenge. Infect. Immun. 682756-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuespert, K., S. Pils, and C. R. Hauck. 2006. CEACAMs: their role in physiology and pathophysiology. Curr. Opin. Cell Biol. 18565-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kyd, J. M., A. W. Cripps, L. A. Novotny, and L. O. Bakaletz. 2003. Efficacy of the 26-kilodalton outer membrane protein and two P5 fimbrin-derived immunogens to induce clearance of nontypeable Haemophilus influenzae from the rat middle ear and lungs as well as from the chinchilla middle ear and nasopharynx. Infect. Immun. 714691-4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mason, K. M., R. S. Munson, Jr., and L. O. Bakaletz. 2005. A mutation in the sap operon attenuates survival of nontypeable Haemophilus influenzae in a chinchilla model of otitis media. Infect. Immun. 73599-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mason, K. M., R. S. Munson, Jr., and L. O. Bakaletz. 2003. Nontypeable Haemophilus influenzae gene expression induced in vivo in a chinchilla model of otitis media. Infect. Immun. 713454-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCaw, S. E., J. Schneider, E. H. Liao, W. Zimmermann, and S. D. Gray-Owen. 2003. Immunoreceptor tyrosine-based activation motif phosphorylation during engulfment of Neisseria gonorrhoeae by the neutrophil-restricted CEACAM3 (CD66d) receptor. Mol. Microbiol. 49623-637. [DOI] [PubMed] [Google Scholar]
- 30.McGillivary, G., W. C. Ray, C. L. Bevins, R. S. Munson, Jr., and L. O. Bakaletz. 2007. A member of the cathelicidin family of antimicrobial peptides is produced in the upper airway of the chinchilla and its mRNA expression is altered by common viral and bacterial co-pathogens of otitis media. Mol. Immunol. 442446-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyamoto, N., and L. O. Bakaletz. 1997. Kinetics of the ascension of NTHi from the nasopharynx to the middle ear coincident with adenovirus-induced compromise in the chinchilla. Microb. Pathog. 23119-126. [DOI] [PubMed] [Google Scholar]
- 32.Miyamoto, N., and L. O. Bakaletz. 1996. Selective adherence of non-typeable Haemophilus influenzae (NTHi) to mucus or epithelial cells in the chinchilla Eustachian tube and middle ear. Microb. Pathog. 21343-356. [DOI] [PubMed] [Google Scholar]
- 33.Muenzner, P., C. Dehio, T. Fujiwara, M. Achtman, T. F. Meyer, and S. D. Gray-Owen. 2000. Carcinoembryonic antigen family receptor specificity of Neisseria meningitidis Opa variants influences adherence to and invasion of proinflammatory cytokine-activated endothelial cells. Infect. Immun. 683601-3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muenzner, P., M. Naumann, T. F. Meyer, and S. D. Gray-Owen. 2001. Pathogenic Neisseria trigger expression of their carcinoembryonic antigen-related cellular adhesion molecule 1 (CEACAM1; previously CD66a) receptor on primary endothelial cells by activating the immediate early response transcription factor, nuclear factor-kappaB. J. Biol. Chem. 27624331-24340. [DOI] [PubMed] [Google Scholar]
- 35.Najjar, S. M. 2002. Regulation of insulin action by CEACAM1. Trends Endocrinol. Metab. 13240-245. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura, A., T. F. DeMaria, D. J. Lim, and C. A. van Blitterswijk. 1991. Primary culture of chinchilla middle ear epithelium. Ann. Otol. Rhinol. Laryngol. 100774-782. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura, A., T. F. DeMaria, C. van Blitterswijk, and D. J. Lim. 1992. Effect of endotoxin on cultured chinchilla middle ear epithelium. Ann. Otol. Rhinol. Laryngol. 101607-611. [DOI] [PubMed] [Google Scholar]
- 38.Novotny, L. A., J. A. Jurcisek, F. Godfroid, J. T. Poolman, P. A. Denoel, and L. O. Bakaletz. 2006. Passive immunization with human anti-protein D antibodies induced by polysaccharide protein D conjugates protects chinchillas against otitis media after intranasal challenge with Haemophilus influenzae. Vaccine 244804-4811. [DOI] [PubMed] [Google Scholar]
- 39.Novotny, L. A., J. A. Jurcisek, M. E. Pichichero, and L. O. Bakaletz. 2000. Epitope mapping of the outer membrane protein P5-homologous fimbrin adhesin of nontypeable Haemophilus influenzae. Infect. Immun. 682119-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Novotny, L. A., K. M. Mason, and L. O. Bakaletz. 2005. Development of a chinchilla model to allow direct, continuous, biophotonic imaging of bioluminescent nontypeable Haemophilus influenzae during experimental otitis media. Infect. Immun. 73609-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Novotny, L. A., M. E. Pichichero, P. A. Denoel, C. Neyt, S. Vanderschrick, G. Dequesne, and L. O. Bakaletz. 2002. Detection and characterization of pediatric serum antibody to the OMP P5-homologous adhesin of nontypeable Haemophilus influenzae during acute otitis media. Vaccine 203590-3597. [DOI] [PubMed] [Google Scholar]
- 42.Odin, P., M. Asplund, C. Busch, and B. Obrink. 1988. Immunohistochemical localization of cellCAM 105 in rat tissues: appearance in epithelia, platelets, and granulocytes. J. Histochem. Cytochem. 36729-739. [DOI] [PubMed] [Google Scholar]
- 43.Odin, P., and B. Obrink. 1987. Quantitative determination of the organ distribution of the cell adhesion molecule cell-CAM 105 by radioimmunoassay. Exp. Cell Res. 1711-15. [DOI] [PubMed] [Google Scholar]
- 44.Prall, F., P. Nollau, M. Neumaier, H. D. Haubeck, Z. Drzeniek, U. Helmchen, T. Loning, and C. Wagener. 1996. CD66a (BGP), an adhesion molecule of the carcinoembryonic antigen family, is expressed in epithelium, endothelium, and myeloid cells in a wide range of normal human tissues. J. Histochem. Cytochem. 4435-41. [DOI] [PubMed] [Google Scholar]
- 45.Reddy, M. S., J. M. Bernstein, T. F. Murphy, and H. S. Faden. 1996. Binding between outer membrane proteins of nontypeable Haemophilus influenzae and human nasopharyngeal mucin. Infect. Immun. 641477-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sarantis, H., and S. D. Gray-Owen. 2007. The specific innate immune receptor CEACAM3 triggers neutrophil bactericidal activities via a Syk kinase-dependent pathway. Cell. Microbiol. 92167-2180. [DOI] [PubMed] [Google Scholar]
- 47.Schmitter, T., F. Agerer, L. Peterson, P. Munzner, and C. R. Hauck. 2004. Granulocyte CEACAM3 is a phagocytic receptor of the innate immune system that mediates recognition and elimination of human-specific pathogens. J. Exp. Med. 19935-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sirakova, T., P. E. Kolattukudy, D. Murwin, J. Billy, E. Leake, D. Lim, T. DeMaria, and L. Bakaletz. 1994. Role of fimbriae expressed by nontypeable Haemophilus influenzae in pathogenesis of and protection against otitis media and relatedness of the fimbrin subunit to outer membrane protein A. Infect. Immun. 622002-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sundberg, U., N. Beauchemin, and B. Obrink. 2004. The cytoplasmic domain of CEACAM1-L controls its lateral localization and the organization of desmosomes in polarized epithelial cells. J. Cell Sci. 1171091-1104. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki, K., and L. O. Bakaletz. 1994. Synergistic effect of adenovirus type 1 and nontypeable Haemophilus influenzae in a chinchilla model of experimental otitis media. Infect. Immun. 621710-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toleman, M., E. Aho, and M. Virji. 2001. Expression of pathogen-like Opa adhesins in commensal Neisseria: genetic and functional analysis. Cell. Microbiol. 333-44. [DOI] [PubMed] [Google Scholar]
- 52.Virji, M., K. Makepeace, D. J. Ferguson, and S. M. Watt. 1996. Carcinoembryonic antigens (CD66) on epithelial cells and neutrophils are receptors for Opa proteins of pathogenic neisseriae. Mol. Microbiol. 22941-950. [DOI] [PubMed] [Google Scholar]
- 53.Virji, M., S. M. Watt, S. Barker, K. Makepeace, and R. Doyonnas. 1996. The N-domain of the human CD66a adhesion molecule is a target for Opa proteins of Neisseria meningitidis and Neisseria gonorrhoeae. Mol. Microbiol. 22929-939. [DOI] [PubMed] [Google Scholar]
- 54.Wang, J., S. D. Gray-Owen, A. Knorre, T. F. Meyer, and C. Dehio. 1998. Opa binding to cellular CD66 receptors mediates the transcellular traversal of Neisseria gonorrhoeae across polarized T84 epithelial cell monolayers. Mol. Microbiol. 30657-671. [DOI] [PubMed] [Google Scholar]
- 55.Wong, R. L., D. Mytych, S. Jacobs, R. Bordens, and S. J. Swanson. 1997. Validation parameters for a novel biosensor assay which simultaneously measures serum concentrations of a humanized monoclonal antibody and detects induced antibodies. J. Immunol. Methods 2091-15. [DOI] [PubMed] [Google Scholar]
- 56.Yu, Q., E. M. Chow, H. Wong, J. Gu, O. Mandelboim, S. D. Gray-Owen, and M. A. Ostrowski. 2006. CEACAM1 (CD66a) promotes human monocyte survival via a phosphatidylinositol 3-kinase- and AKT-dependent pathway. J. Biol. Chem. 28139179-39193. [DOI] [PubMed] [Google Scholar]