Abstract
SMC (structural maintenance of chromosomes) proteins play fundamental roles in various aspects of chromosome organization and dynamics, including repair of DNA damage. Mutant strains of Mycobacterium smegmatis and Mycobacterium tuberculosis defective in SMC were constructed. Surprisingly, inactivation of smc did not result in recognizable phenotypes in hallmark assays characteristic for the function of these genes. This is in contrast to data for smc null mutants in other species.
Most bacterial cells contain a single circular chromosome that is folded and compacted into a structure called the nucleoid (24). Diverse DNA binding proteins are associated with the nucleoid and take part in chromosome organization to assist in a variety of complex processes such as replication, recombination, repair, modification, and transcription (8). Among these proteins are the SMC (structural maintenance of chromosomes) proteins that are conserved from prokaryotes to eukaryotes (4, 5, 28). The SMC family proteins are large proteins in the range between 110 and 170 kDa and share common principles in domain organization: globular N- and C-terminal domains which are connected by two long coiled-coil domains, separated by a globular hinge domain of approximately 150 amino acids in length (12).
Eukaryotes possess at least six distinct SMCs that participate in chromosome condensation, sister chromatid cohesion, DNA repair, and gene dosage compensation (16, 19). Pairs of SMC proteins form antiparallel heterodimers which assemble with accessory proteins into complexes, such as the condensin complex (SMC2 and SMC4), which condenses the chromosomes during mitosis, and the cohesin complex (SMC1 and SMC3), which mediates sister chromatid cohesion. Another complex composed of SMC5 and SMC6 is involved in the cellular response to DNA damage. Genetic and biochemical evidence suggests that cohesin and the SMC5/6 complex cooperate in the recombinational repair of double-strand breaks (17). The cohesin complex assists in homologous recombination by holding sister chromatids together in the vicinity of the double-strand breaks (26). The function of the SMC5/6 complex was first recognized by the increased sensitivity of Schizosaccharomyces pombe SMC6 mutants to UV light and ionizing radiation (21). Recent investigations of the SMC5/6 complex in yeasts, plants, and human cells have shown that deletion of components of this complex leads to an increased sensitivity towards DNA-damaging agents (22).
In contrast to the multiple SMC proteins present in eukaryotes, most bacteria possess only a single SMC (28). This single SMC protein is proposed to carry out several of the functions divided between specialized SMC complexes in eukaryotic cells. Bacterial SMC proteins form antiparallel homodimers through interactions in the hinge region, resulting in symmetric molecules where both ends contain an ATPase and a DNA binding domain (10). The gammaproteobacteria (such as Escherichia coli) possess the MukB protein instead of SMC. MukB is distantly related to SMC with structural and functional similarity (20). SMC from Bacillus subtilis or Caulobacter crescentus functionally resembles eukaryotic SMCs. The proteins are not essential, but null mutants of SMC generate anucleate cells (“titan cells”) and display aberrant nucleoid formation (decondensed chromosomes) (2, 15, 31). In addition, deletion of SMC results in increased susceptibility to DNA-damaging agents (7).
Little is known about chromosome stability, organization, and partitioning in mycobacteria. Mycobacterium tuberculosis persists for decades in its host during latent infection (30). It is thus of interest to investigate the mechanisms which contribute to the capability of the bacterium to stabilize its genome during persistence and ensure proper cell division following reactivation. Bioinformatic analyses indicated that all mycobacterial genomes encode a single SMC homologue. A homologous open reading frame (ORF) is also present in Mycobacterium leprae, a bacterium that has lost various gene functions during reductive genomic evolution (6).
Mycobacterium smegmatis smc (MSMEG2422) encodes an 1,195-amino-acid polypeptide with a calculated molecular mass of 129.7 kDa. The protein shares all structural motifs conserved in members of the SMC family as determined by Clustal W multiple sequence alignments and InterPro Scan protein motif searches. Homologous sequences are restricted to the head, hinge, and tail domains. The three domains are connected by two long heptad repeat regions predicted to form coiled coils. Overall, M. smegmatis SMC shares 74% identity with the 1,205-amino-acid SMC sequence of M. tuberculosis and 55% identity with the 1,186-amino-acid SMC sequence of B. subtilis. Eukaryotic SMC proteins functionally interact with other proteins, and SMC of B. subtilis was found to function in concert with two proteins called ScpA and ScpB (11). Both M. tuberculosis and M. smegmatis have homologues of these interacting proteins, suggesting that mycobacterial SMC is functional (ScpA homologues, Rv1709/MSMEG3748; ScpB homologues, Rv1710/MSMEG3749; sequences were obtained from The Institute for Genomic Research website and TubercuList of Institut Pasteur, respectively).
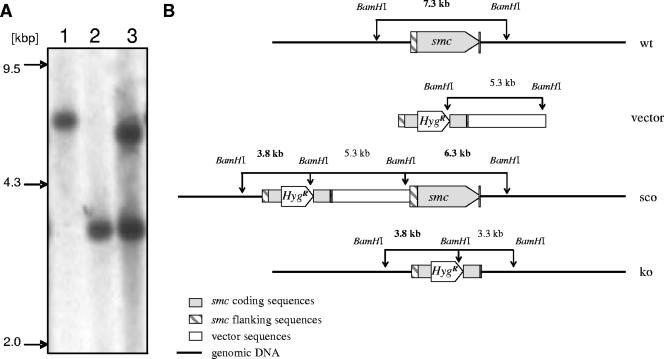
To study the function of mycobacterial SMC, we generated an smc-null mutant in Mycobacterium smegmatis, an established model for genetic and biochemical studies in mycobacteria (1, 14). The mutant was constructed by allelic-exchange mutagenesis (14, 23). Using genomic DNA as template, a 2-kb deletion allele was constructed by joining PCR products that contain parts of the ORF flanked by upstream and downstream sequences. The resulting deletion allele lacks bp 747 to 2660 of the 3,588-bp ORF. To allow proper selection of gene deletion mutants, a hygromycin resistance cassette was inserted between the two fragments, resulting in the deletion allele smc::hyg. Transformation and counterselection procedures for M. smegmatis were performed at room temperature. Transformants were plated on medium containing hygromycin to select for homologous recombination of the knockout vector into the genome. Southern blot analysis revealed a clone that displayed a pattern indicative of a single-crossover event, i.e., tandem arrangement of the deletion allele upstream of the functional smc allele (designated 5′ sco). The single-crossover clone was grown for 5 days in liquid medium at room temperature. Subsequently, the bacteria were plated on selective 7H10 agar containing streptomycin and hygromycin to select against maintenance of knockout vector sequences which contain the wild-type rpsL gene. Southern blot analyses demonstrated that in 11 of 24 analyzed clones, the wild-type smc gene was replaced by the deletion allele, resulting in smc knockout mutants (Fig. 1). All selection steps were set off with bacteria taken from frozen stocks to ensure that no suppressor mutations arose due to extensive passaging.
FIG. 1.
Generation of M. smegmatis smc mutant. (A) Southern blot analysis. Genomic DNA from M. smegmatis wild type (lane 1), smc mutant (lane 2), and the smc single-crossover mutant (lane 3) was digested with BamHI and probed with a 685-bp PvuII gene fragment containing the 5′ flanking region of the smc gene. The presence of a single 3.8-kbp fragment in the mutant strain instead of a 7.3-kbp fragment observed in the parental strain demonstrates successful deletion of smc coding sequences. (B) Schematic illustration of the smc locus and the Southern blot strategy. Shown are the genomic organization of the wild type (wt), the knockout vector that contains the deletion allele smc::hyg (vector), the single-crossover genotype (sco), and the mutated genomic smc region in the knockout mutant (ko). Fragments detected by the probe specific for the 5′ flanking region are shown in bold letters.
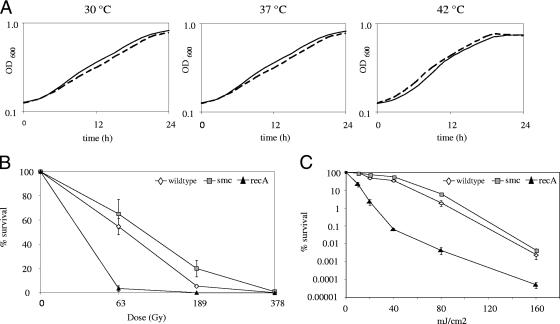
One prominent phenotype of previously described smc null mutants is temperature sensitivity (2, 15, 31). As many SMC mutants have distinctive growth defects at high growth rates (2), we analyzed the in vitro growth characteristics in 7H9 broth in comparison to the parental wild-type strain. 7H9 medium is known to support optimal in vitro growth of mycobacteria. In addition to growth at the standard incubation temperature (37°C), we assessed the growth rate at lower (30°C) and higher (42°C) temperatures. In contrast to smc null mutant phenotypes reported for B. subtilis and C. crescentus, the growth rates of the mutant and the wild type were similar in M. smegmatis at the various temperatures investigated (Fig. 2A). Doubling times in logarithmic phase were nearly identical, indicating that smc deletion does not affect in vitro growth of M. smegmatis. Additionally, the wild type and the mutant were indistinguishable with respect to colony morphology; when grown on 7H10 agar, both strains showed the typical features of mycobacterial colonies with rough dry surfaces and irregular edges (data not shown).
FIG. 2.
Analysis of M. smegmatis smc mutant strain. (A) Growth of M. smegmatis wild-type strain (solid lines) and smc mutant strain (dashed lines) at various temperatures. OD600, optical density at 600 nm. (B) Survival following exposure to ionizing radiation. (C) Survival following exposure to UV.
To assess if SMC deficiency leads to an increase in mutation frequency, the frequencies of spontaneous resistance to rifampin were determined. Four independent cultures of the parental wild-type strain and of the mutant strain were grown in 7H9 broth to mid-log phase, and aliquots were plated on 7H10 plates containing rifampin (175 μg/ml). To calculate the spontaneous mutation frequency, CFU were determined in parallel by plating serial dilutions on nonselective LB medium. Mutation frequencies were comparable in wild-type (3.1 × 10−8 ± 1.5 × 10−8) and mutant (3.8 × 10−8 ± 1.0 × 10−8; P = 0.68; n = 4; Student's t test) strains, indicating that SMC deficiency does not result in a mutator phenotype in M. smegmatis.
DNA gyrase, which generates negative supercoiling, and topoisomerase I, which prevents excessive negative supercoiling by DNA gyrase, are essential to maintain the optimal topological state of DNA in the cell (3). Previous investigations have indicated that SMC proteins mediate chromosome compaction by contributing to the introduction of negative supercoils into DNA (29). This is corroborated by the observation that smc and mukB mutants are hypersusceptible to gyrase inhibitors (25), while loss of MukB function can be partially compensated for by reducing topoisomerase I activity (29). To investigate if SMC interferes with DNA supercoiling in M. smegmatis, we determined MICs towards the gyrase inhibitor ofloxacin using the E-test (AB Biodisk). In contrast to findings made in other organisms (25), M. smegmatis wild-type and smc mutant strains displayed identical drug susceptibilities to ofloxacin, with MICs of 0.19 μg/ml.
SMC is known to participate in the repair of DNA double-strand breaks in various organisms (22, 27), prompting us to examine the ability of the SMC mutant strain to survive treatment with ionizing radiation. Mid-log-phase cultures were irradiated (0 to 378 Gy), and CFU were determined. As DNA double-strand breaks are also repaired by homologous recombination repair (13), we included a recA mutant strain as a control. In contrast to the recA mutant strain, no major difference in survival between the wild-type strain and the smc mutant strain was found (Fig. 2B). The eukaryotic SMC5/6 complex has been described to act in a second pathway for postreplicative repair of UV-induced lesions, which is different from the standard nucleotide excision repair pathway (18). In order to assess a possible role of mycobacterial SMC in repair of UV-induced lesions, we investigated the sensitivity of the SMC mutant strain to UV irradiation. Three independent cultures of each strain were grown in 7H9 medium until late log phase (optical density, 1 to 2). Serial dilutions of each strain were plated on LB agar. Subsequently, the plates were exposed to different UV doses (0 to 160 mJ/cm2, 254 nm) and the number of CFU were determined. Compared to the recA mutant strain, which was impaired in survival, the wild-type strain and the smc mutant strain displayed similar susceptibilities to UV (Fig. 2C). Investigation of susceptibility to mitomycin C, a compound that damages DNA by generating intrastrand cross-links, revealed no difference between the smc mutant strain and the wild-type strain (data not shown). In summary, our data provide evidence that mycobacterial SMC has, if at all, a minor role in DNA repair.
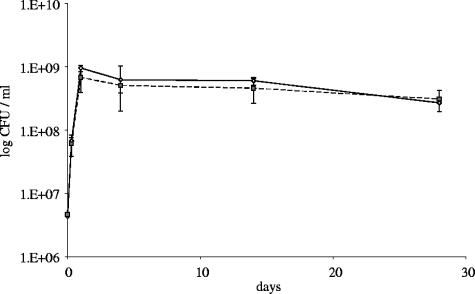
Some genes are not essential for exponential growth but are prerequisites for remaining viable during stationary phase (9). In order to gain insight into a possible role of SMC in long-term survival of M. smegmatis, we compared CFU counts of the M. smegmatis wild type and the smc mutant grown for 28 days in 7H9 broth. Viable counts of stationary cultures were similar for the two strains (Fig. 3), suggesting that SMC is not essential for long-term survival during stationary phase.
FIG. 3.
Long-term survival of M. smegmatis wild-type strain (solid line) and smc mutant strain (dashed line).
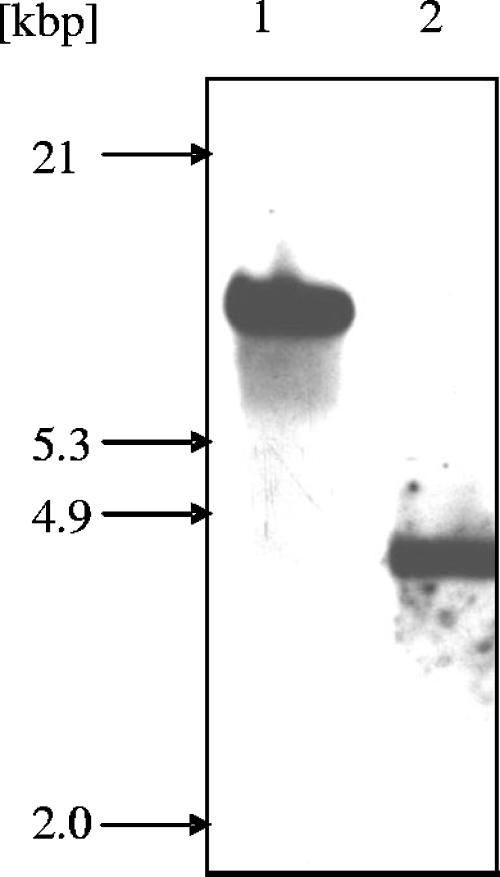
To study whether the findings obtained in the model organism M. smegmatis hold true for its pathogenic relatives, we generated an M. tuberculosis smc mutant. A deletion allele lacking 2,121 bp of the 3,618-bp smc ORF (the resulting mutant lacks bp 553 to 2674 of the ORF) was constructed by allelic-replacement mutagenesis as outlined for M. smegmatis above. Disruption of smc was verified by Southern blot analysis (Fig. 4). Growth of the mutant at 37°C in 7H9 broth supplemented with oleic acid-albumin-dextrose-catalase was not impaired, indicating that interruption of smc does not affect in vitro growth (data not shown). Survival in stationary phase was determined by comparing CFU counts of the M. tuberculosis wild-type and smc mutant strains grown for 83 days in 7H9 broth supplemented with oleic acid-albumin-dextrose-catalase. Both strains reach viable cell counts of about 1 × 108 CFU/ml after growth for 9 days, which for both strains dropped to approximately 3 × 107 CFU/ml after 83 days of incubation, suggesting that SMC is not required for long-term survival of M. tuberculosis (data not shown).
FIG. 4.
Generation of M. tuberculosis smc mutant. Genomic DNA from M. tuberculosis wild-type (lane 1) and smc mutant (lane 2) strains was digested with BamHI and probed with a 935-bp NdeI gene fragment containing the 5′ flanking region of the smc gene. The presence of a single 4.3-kbp fragment instead of an 11.1-kbp fragment as seen in the parental strain demonstrates successful deletion of smc coding sequences.
In this study smc mutant strains of M. smegmatis and M. tuberculosis were constructed. In contrast to previous data obtained from other bacterial smc null mutants, SMC deficiency did not result in apparent phenotypes. This is surprising given the crucial role of SMC proteins in chromosome dynamics and DNA repair in all organisms analyzed so far (12, 17, 19). Construction and analysis of scpA and scpB knockout mutants will be subjects of future investigations. These mutants eventually corroborate the results obtained with the smc mutants or reveal a possible additional, SMC-independent involvement of these proteins in mycobacterial chromosome organization.
Taken together, our observations suggest that maintenance of mycobacterial chromosome organization differs from that of previously described models. Further investigations of mycobacteria are likely to provide insights into new mechanisms which maintain genome integrity and ensure persistence.
Acknowledgments
This work was supported in part by grants from the University of Zurich (to B.S.), the Swiss National Science Foundation (to P.S. and E.C.B.; BO-3200-68488), and the European Community (to B.S. and E.C.B.; CSI_LTB, LSHP-CT-2007-037235).
Footnotes
Published ahead of print on 2 November 2007.
REFERENCES
- 1.Acharya, N., and U. Varshney. 2002. Biochemical properties of single-stranded DNA-binding protein from Mycobacterium smegmatis, a fast-growing mycobacterium and its physical and functional interaction with uracil DNA glycosylases. J. Mol. Biol. 3181251-1264. [DOI] [PubMed] [Google Scholar]
- 2.Britton, R. A., D. C.-H. Lin, and A. D. Grossman. 1998. Characterization of a prokaryotic SMC protein involved in chromosome partitioning. Genes Dev. 121254-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Champoux, J. J. 2001. DNA topoisomerases: structure, function and mechanism. Annu. Rev. Biochem. 70369-413. [DOI] [PubMed] [Google Scholar]
- 4.Cobbe, N., and M. M. Heck. 2004. The evolution of SMC proteins: phylogenetic analysis and structural implications. Mol. Biol. Evol. 21332-347. [DOI] [PubMed] [Google Scholar]
- 5.Cobbe, N., and M. M. S. Heck. 2000. Review: SMCs in the world of chromosome biology—from prokaryotes to higher eukaryotes. J. Struct. Biol. 129123-143. [DOI] [PubMed] [Google Scholar]
- 6.Cole, S. T., K. Eiglmeier, J. Parkhill, K. D. James, N. R. Thomson, P. R. Wheeler, N. Honore, T. Garnier, C. Churcher, D. Harris, K. Mungall, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. M. Davies, K. Devlin, S. Duthoy, T. Feltwell, A. Fraser, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, C. Lacroix, J. Maclean, S. Moule, L. Murphy, K. Oliver, M. A. Quail, M.-A. Rajandream, K. M. Rutherford, S. Rutter, K. Seeger, S. Simon, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, K. Taylor, S. Whitehead, J. R. Woodward, and B. G. Barrell. 2001. Massive gene decay in the leprosy bacillus. Nature 4091007-1011. [DOI] [PubMed] [Google Scholar]
- 7.Dervyn, E., M. F. Noirot-Gros, P. Mervelet, S. McGovern, S. D. Ehrlich, P. Polard, and P. Noirot. 2004. The bacterial condensin/cohesin-like protein complex acts in DNA repair and regulation of gene expression. Mol. Microbiol. 511629-1640. [DOI] [PubMed] [Google Scholar]
- 8.Frenkiel-Krispin, D., and A. Minsky. 2006. Nucleoid organization and the maintenance of DNA integrity in E. coli, B. subtilis and D. radiodurans. J. Struct. Biol. 156311-319. [DOI] [PubMed] [Google Scholar]
- 9.Hampshire, T., S. Soneji, J. Bacon, B. W. James, J. Hinds, K. Laing, R. A. Stabler, P. D. Marsh, and P. D. Butcher. 2004. Stationary phase gene expression of Mycobacterium tuberculosis following a progressive nutrient depletion: a model for persistent organisms? Tuberculosis 84228-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirano, M., and T. Hirano. 2002. Hinge-mediated dimerization of SMC protein is essential for its dynamic interaction with DNA. EMBO J. 215733-5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirano, M., and T. Hirano. 2004. Positive and negative regulation of SMC-DNA interactions by ATP and accessory proteins. EMBO J. 232664-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirano, T. 2005. SMC proteins and chromosome mechanics: from bacteria to humans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 360507-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishino, Y., T. Nishino, and K. Morikawa. 2006. Mechanisms of maintaining genetic stability by homologous recombination. Chem. Rev. 106324-339. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs, W. R., Jr., G. V. Kalpana, J. D. Cirillo, L. Pascopella, S. B. Snapper, R. A. Udani, W. Jones, R. G. Barletta, and B. R. Bloom. 1991. Genetic systems for mycobacteria. Methods Enzymol. 204537-555. [DOI] [PubMed] [Google Scholar]
- 15.Jensen, R. B., and L. Shapiro. 1999. The Caulobacter crescentus smc gene is required for cell cycle progression and chromosome segregation. Proc. Natl. Acad. Sci. USA 9610661-10666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jessberger, R. 2002. The many functions of SMC proteins in chromosome dynamics. Nat. Rev. Mol. Cell Biol. 3767-778. [DOI] [PubMed] [Google Scholar]
- 17.Lehmann, A. R. 2005. The role of SMC proteins in the responses to DNA damage. DNA Repair 4309-314. [DOI] [PubMed] [Google Scholar]
- 18.Lehmann, A. R., M. Walicka, D. J. Griffiths, J. M. Murray, F. Z. Watts, S. McCready, and A. M. Carr. 1995. The rad18 gene of Schizosaccharomyces pombe defines a new subgroup of the SMC superfamily involved in DNA repair. Mol. Cell. Biol. 157067-7080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Losada, A., and T. Hirano. 2005. Dynamic molecular linkers of the genome: the first decade of SMC proteins. Genes Dev. 191269-1287. [DOI] [PubMed] [Google Scholar]
- 20.Melby, T. E., C. N. Ciampaglio, G. Briscoe, and H. P. Erickson. 1998. The symmetrical structure of structural maintenance of chromosomes (SMC) and MukB proteins: long, antiparallel coiled coils, folded at a flexible hinge. J. Cell Biol. 1421595-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nasim, A., and B. P. Smith. 1975. Genetic control of radiation sensitivity in Schizosaccharomyces pombe. Genetics 79573-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Potts, P. R., M. H. Porteus, and H. Yu. 2006. Human SMC5/6 complex promotes sister chromatid homologous recombination by recruiting the SMC1/3 cohesin complex to double-strand breaks. EMBO J. 253377-3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sander, P., A. Meier, and E. C. Böttger. 1995. rpsL+: a dominant selectable marker for gene replacement in mycobacteria. Mol. Microbiol. 16991-1000. [DOI] [PubMed] [Google Scholar]
- 24.Sandman, K., S. L. Pereira, and J. N. Reeve. 1998. Diversity of prokaryotic chromosomal proteins and the origin of the nucleosome. Cell. Mol. Life Sci. 541350-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sawitzke, J. A., and S. Austin. 2000. Suppression of chromosome segregation defects of Escherichia coli muk mutants by mutations in topoisomerase I. Proc. Natl. Acad. Sci. USA 971671-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schär, P., M. Fasi, and R. Jessberger. 2004. SMC1 coordinates DNA double-strand break repair pathways. Nucleic Acids Res. 323921-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sjogren, C., and K. Nasmyth. 2001. Sister chromatid cohesion is required for postreplicative double-strand break repair in Saccharomyces cerevisiae. Curr. Biol. 11991-995. [DOI] [PubMed] [Google Scholar]
- 28.Soppa, J. 2001. Prokaryotic structural maintenance of chromosomes (SMC) proteins: distribution, phylogeny, and comparison with MukBs and additional prokaryotic and eukaryotic coiled-coil proteins. Gene 278253-264. [DOI] [PubMed] [Google Scholar]
- 29.Tadesse, S., and P. L. Graumann. 2006. Differential and dynamic localization of topoisomerases in Bacillus subtilis. J. Bacteriol. 1883002-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tufariello, J. M., J. Chan, and J. L. Flynn. 2003. Latent tuberculosis: mechanisms of host and bacillus that contribute to persistent infection. Lancet Infect. Dis. 3578-590. [DOI] [PubMed] [Google Scholar]
- 31.Volkov, A., J. Mascarenhas, C. Andrei-Selmer, H. D. Ulrich, and P. L. Graumann. 2003. A prokaryotic condensin/cohesin-like complex can actively compact chromosomes from a single position on the nucleoid and binds to DNA as a ring-like structure. Mol. Cell. Biol. 235638-5650. [DOI] [PMC free article] [PubMed] [Google Scholar]