Abstract
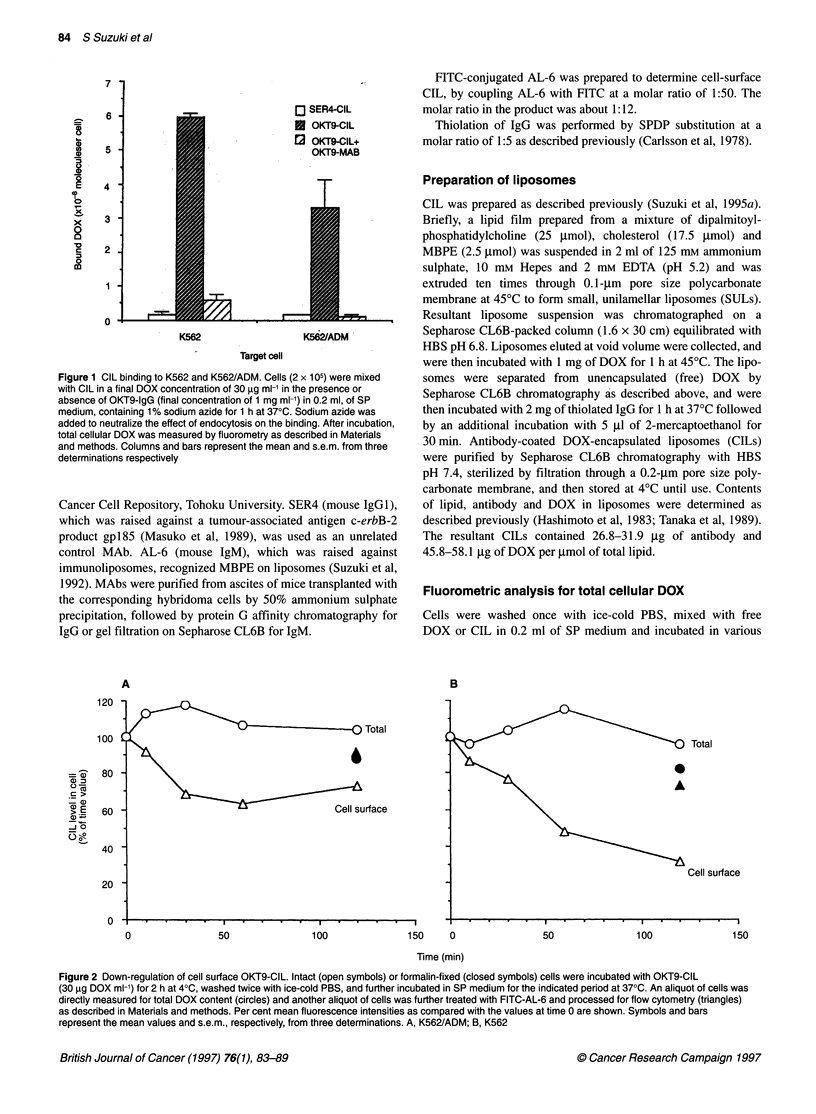
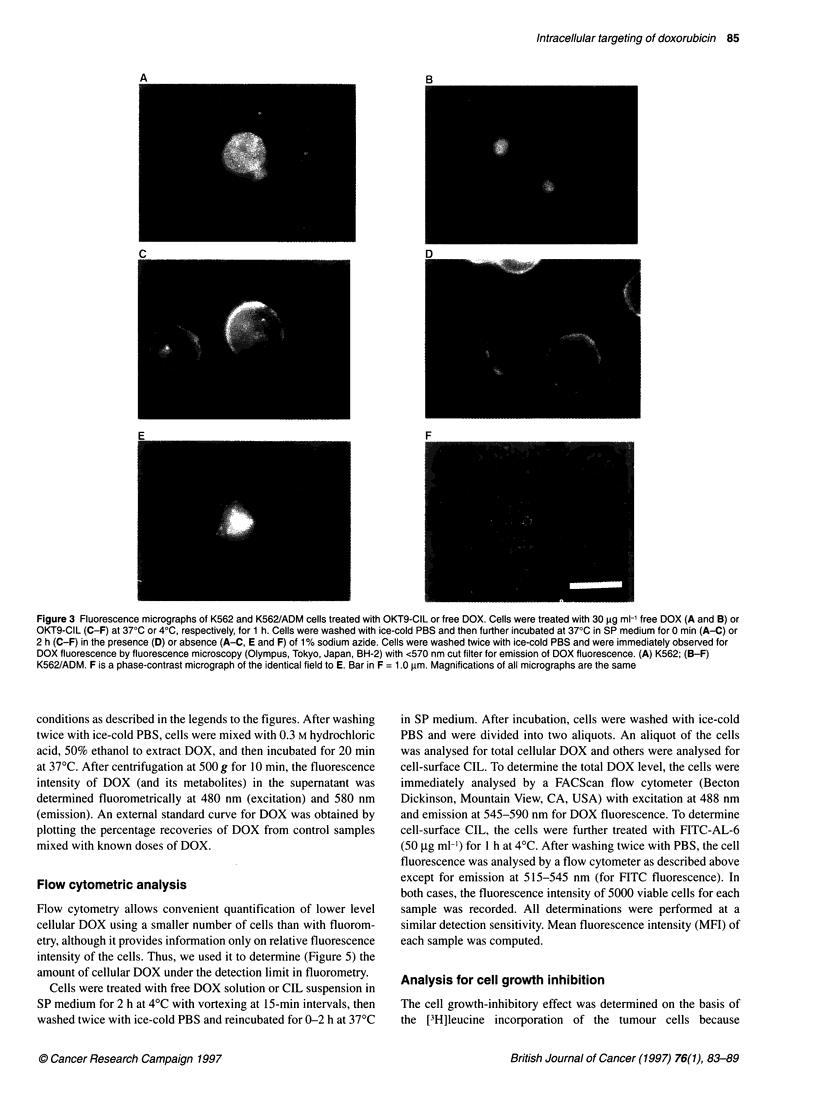
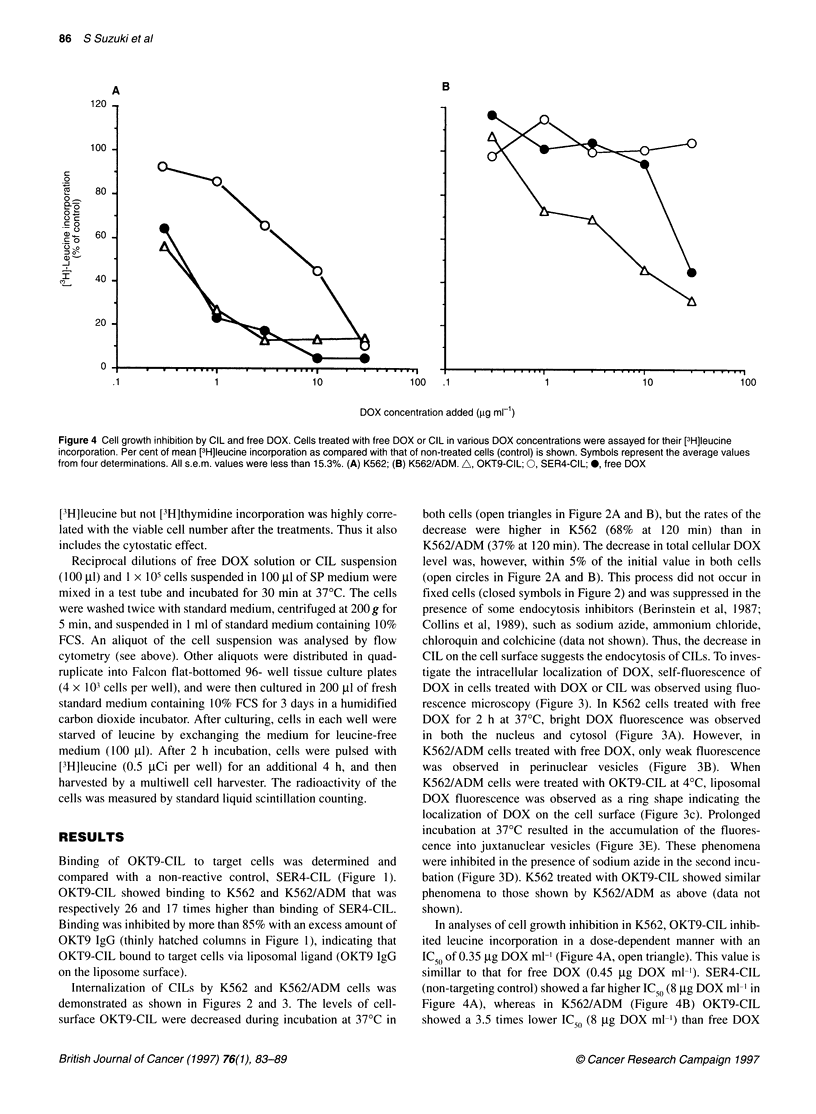
Using a doxorubicin-resistant subline (K562/ADM) of human leukaemia K562 cells (Tsuruo et al, 1986), the effect of immunoliposomes that targeted a cellular transferrin receptor (TFR) was examined by neutralization of doxorubicin (DOX) resistance. OKT9-CIL, prepared by conjugation of DOX-encapsulated liposome with an anti-TFR monoclonal antibody, OKT9 (Aisenberg and Wilkes, 1980), showed similar binding to both K562 and K562/ADM. Although an 80-fold higher sensitivity to free DOX on cell growth inhibition in K562 than in K562/ADM was found, the difference was clearly diminished after OKT9-CIL treatment through the increased sensitivity of K562/ADM. The cellular DOX level 30 min after the exposure of free DOX was 45-fold lower in K562/ADM than in K562, whereas nearly equivalent DOX levels were detected in K562 and K562/ADM after OKT9-CIL treatment. In addition, DOX in K562/ADM in the free DOX treatment was efficiently excreted by 54% within 120 min of incubation, whereas almost all DOX supplied by OKT9-CIL remained uncleared. Fluorescence microscopic observation showed that OKT9-CIL was internalized into juxtanuclear vesicles in K562/ADM cells. These results suggest that OKT9-CIL has a potency to accumulate DOX, resulting in augmentation of DOX cytotoxicity in DOX-resistant tumour cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berinstein N., Matthay K. K., Papahadjopoulos D., Levy R., Sikic B. I. Antibody-directed targeting of liposomes to human cell lines: role of binding and internalization on growth inhibition. Cancer Res. 1987 Nov 15;47(22):5954–5959. [PubMed] [Google Scholar]
- Carlsson J., Drevin H., Axén R. Protein thiolation and reversible protein-protein conjugation. N-Succinimidyl 3-(2-pyridyldithio)propionate, a new heterobifunctional reagent. Biochem J. 1978 Sep 1;173(3):723–737. doi: 10.1042/bj1730723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins D., Maxfield F., Huang L. Immunoliposomes with different acid sensitivities as probes for the cellular endocytic pathway. Biochim Biophys Acta. 1989 Dec 11;987(1):47–55. doi: 10.1016/0005-2736(89)90453-7. [DOI] [PubMed] [Google Scholar]
- Esserman L., Takahashi S., Rojas V., Warnke R., Levy R. An epitope of the transferrin receptor is exposed on the cell surface of high-grade but not low-grade human lymphomas. Blood. 1989 Dec;74(8):2718–2729. [PubMed] [Google Scholar]
- Gervasoni J. E., Jr, Fields S. Z., Krishna S., Baker M. A., Rosado M., Thuraisamy K., Hindenburg A. A., Taub R. N. Subcellular distribution of daunorubicin in P-glycoprotein-positive and -negative drug-resistant cell lines using laser-assisted confocal microscopy. Cancer Res. 1991 Sep 15;51(18):4955–4963. [PubMed] [Google Scholar]
- Gironès N., Davis R. J. Comparison of the kinetics of cycling of the transferrin receptor in the presence or absence of bound diferric transferrin. Biochem J. 1989 Nov 15;264(1):35–46. doi: 10.1042/bj2640035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros P., Ben Neriah Y. B., Croop J. M., Housman D. E. Isolation and expression of a complementary DNA that confers multidrug resistance. Nature. 1986 Oct 23;323(6090):728–731. doi: 10.1038/323728a0. [DOI] [PubMed] [Google Scholar]
- Hamilton T. A., Wada H. G., Sussman H. H. Identification of transferrin receptors on the surface of human cultured cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6406–6410. doi: 10.1073/pnas.76.12.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. L., Hochhauser D. Mechanisms of multidrug resistance in cancer treatment. Acta Oncol. 1992;31(2):205–213. doi: 10.3109/02841869209088904. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Sugawara M., Endoh H. Coating of liposomes with subunits of monoclonal IgM antibody and targeting of the liposomes. J Immunol Methods. 1983 Aug 26;62(2):155–162. doi: 10.1016/0022-1759(83)90242-9. [DOI] [PubMed] [Google Scholar]
- Hindenburg A. A., Gervasoni J. E., Jr, Krishna S., Stewart V. J., Rosado M., Lutzky J., Bhalla K., Baker M. A., Taub R. N. Intracellular distribution and pharmacokinetics of daunorubicin in anthracycline-sensitive and -resistant HL-60 cells. Cancer Res. 1989 Aug 15;49(16):4607–4614. [PubMed] [Google Scholar]
- Lasic D. D., Frederik P. M., Stuart M. C., Barenholz Y., McIntosh T. J. Gelation of liposome interior. A novel method for drug encapsulation. FEBS Lett. 1992 Nov 9;312(2-3):255–258. doi: 10.1016/0014-5793(92)80947-f. [DOI] [PubMed] [Google Scholar]
- Marquardt D., Center M. S. Drug transport mechanisms in HL60 cells isolated for resistance to adriamycin: evidence for nuclear drug accumulation and redistribution in resistant cells. Cancer Res. 1992 Jun 1;52(11):3157–3163. [PubMed] [Google Scholar]
- Masuko T., Sugahara K., Kozono M., Otsuki S., Akiyama T., Yamamoto T., Toyoshima K., Hashimoto Y. A murine monoclonal antibody that recognizes an extracellular domain of the human c-erbB-2 protooncogene product. Jpn J Cancer Res. 1989 Jan;80(1):10–14. doi: 10.1111/j.1349-7006.1989.tb02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay K. K., Abai A. M., Cobb S., Hong K., Papahadjopoulos D., Straubinger R. M. Role of ligand in antibody-directed endocytosis of liposomes by human T-leukemia cells. Cancer Res. 1989 Sep 1;49(17):4879–4886. [PubMed] [Google Scholar]
- Sadasivan R., Morgan R., Fabian C., Stephens R. Reversal of multidrug resistance in HL-60 cells by verapamil and liposome-encapsulated doxorubicin. Cancer Lett. 1991 May 1;57(2):165–171. doi: 10.1016/0304-3835(91)90211-y. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Masuko T., Takanashi K., Takashio K., Hashimoto Y. Assay of cell surface-bound immunoliposomes using monoclonal antibody reactive with a cross-linking reagent. Chem Pharm Bull (Tokyo) 1992 Jul;40(7):1893–1896. doi: 10.1248/cpb.40.1893. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Watanabe S., Uno S., Tanaka M., Masuko T., Hashimoto Y. Endocytosis does not necessarily augment the cytotoxicity of adriamycin encapsulated in immunoliposomes. Biochim Biophys Acta. 1994 Dec 30;1224(3):445–453. doi: 10.1016/0167-4889(94)90281-x. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Suzuki S., Masuko T., Hashimoto Y. In vitro targeting and cytotoxicity of adriamycin in liposomes bearing monoclonal antibody against rat or human gp125 cell proliferation-associated antigen. Jpn J Cancer Res. 1989 Apr;80(4):380–386. doi: 10.1111/j.1349-7006.1989.tb02323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry A. R., Vigé D., Coughlin S. S., Belli J. A., Dritschilo A., Rahman A. Modulation of doxorubicin resistance in multidrug-resistant cells by liposomes. FASEB J. 1993 Apr 1;7(6):572–579. doi: 10.1096/fasebj.7.6.8097173. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Omary M. B. Human cell surface glycoprotein related to cell proliferation is the receptor for transferrin. Proc Natl Acad Sci U S A. 1981 May;78(5):3039–3043. doi: 10.1073/pnas.78.5.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruo T., Iida-Saito H., Kawabata H., Oh-hara T., Hamada H., Utakoji T. Characteristics of resistance to adriamycin in human myelogenous leukemia K562 resistant to adriamycin and in isolated clones. Jpn J Cancer Res. 1986 Jul;77(7):682–692. [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Tsukagoshi S., Sakurai Y. Increased accumulation of vincristine and adriamycin in drug-resistant P388 tumor cells following incubation with calcium antagonists and calmodulin inhibitors. Cancer Res. 1982 Nov;42(11):4730–4733. [PubMed] [Google Scholar]
- Weissman A. M., Klausner R. D., Rao K., Harford J. B. Exposure of K562 cells to anti-receptor monoclonal antibody OKT9 results in rapid redistribution and enhanced degradation of the transferrin receptor. J Cell Biol. 1986 Mar;102(3):951–958. doi: 10.1083/jcb.102.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Cornwell M. M., Cardarelli C. O., Gottesman M. M., Pastan I. Single cell analysis of daunomycin uptake and efflux in multidrug-resistant and -sensitive KB cells: effects of verapamil and other drugs. Cancer Res. 1986 Nov;46(11):5941–5946. [PubMed] [Google Scholar]