Abstract
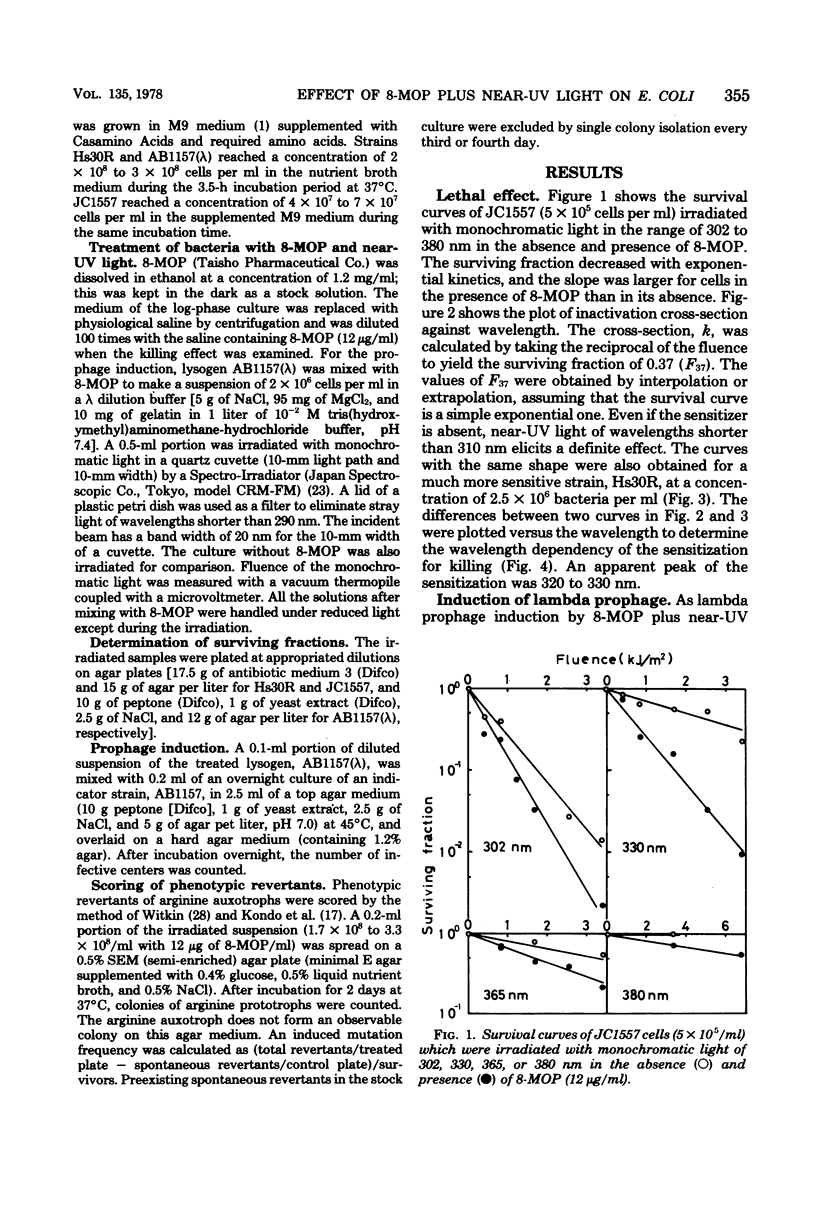
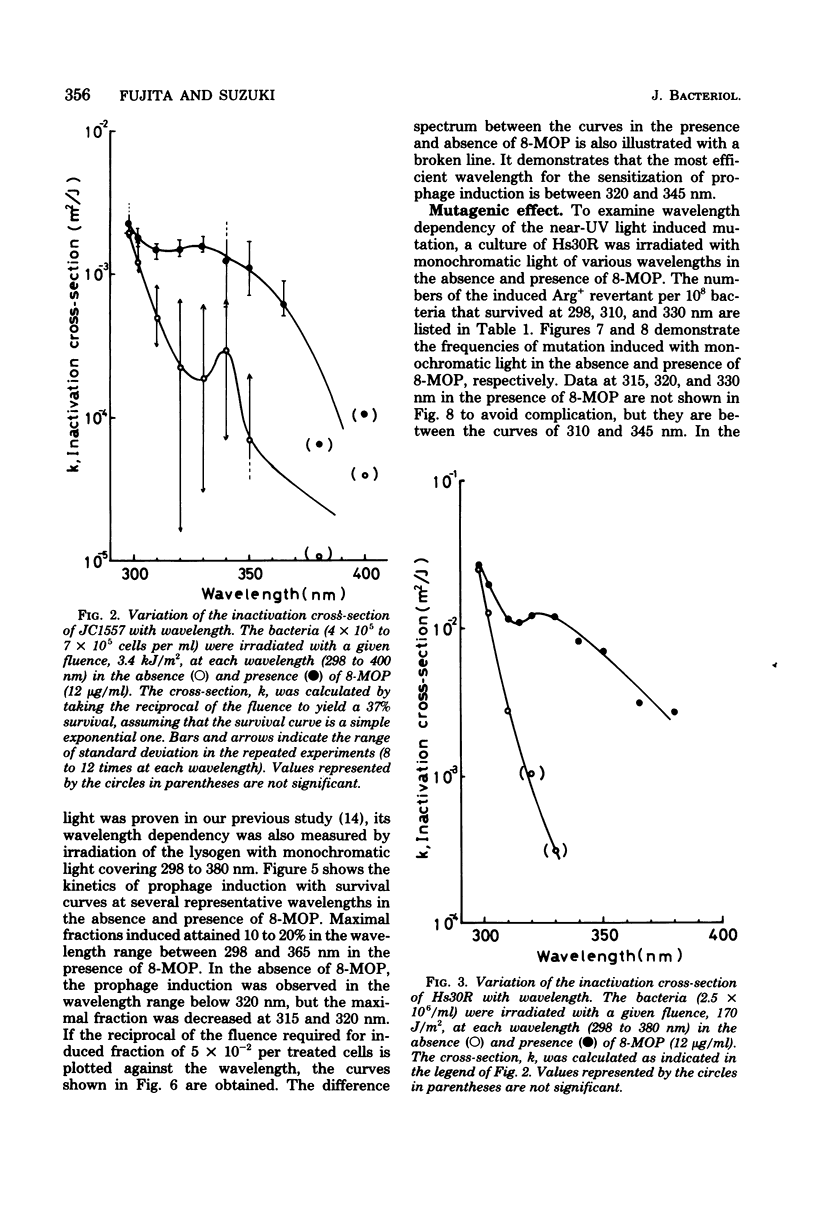
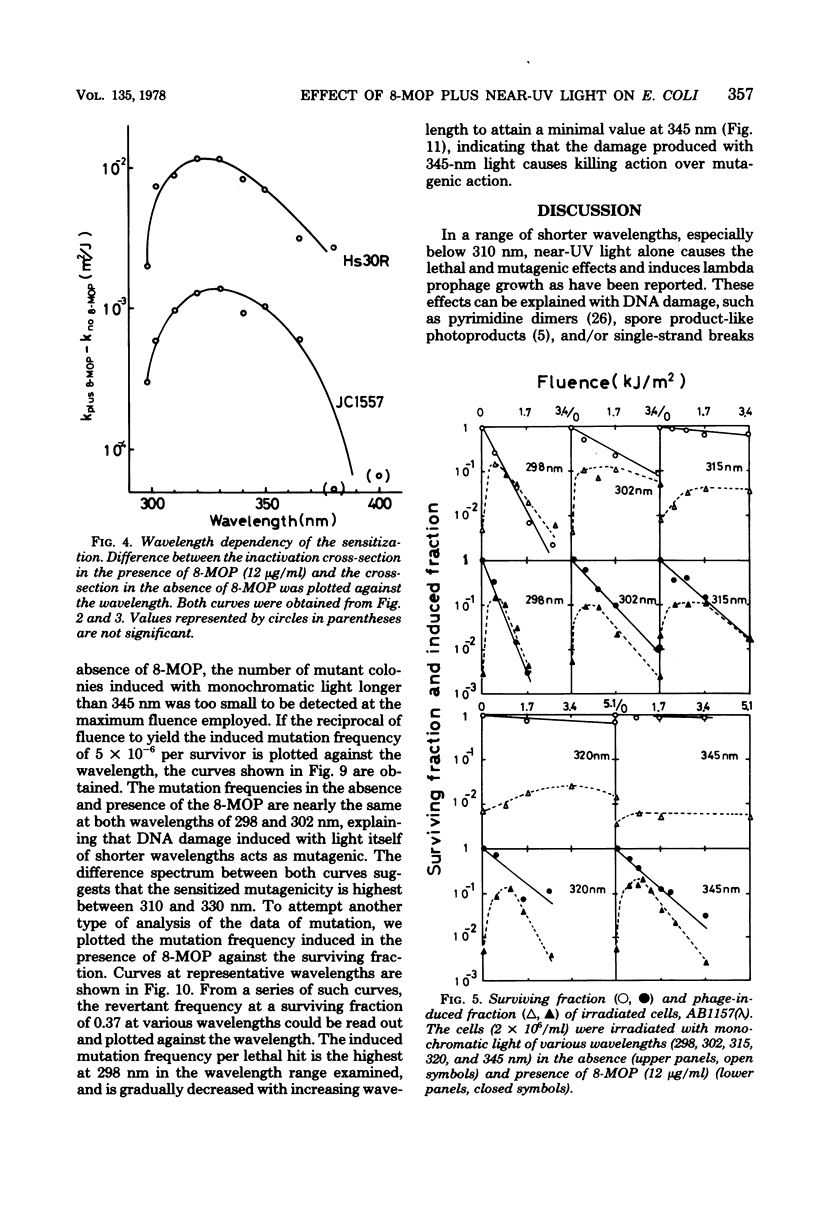
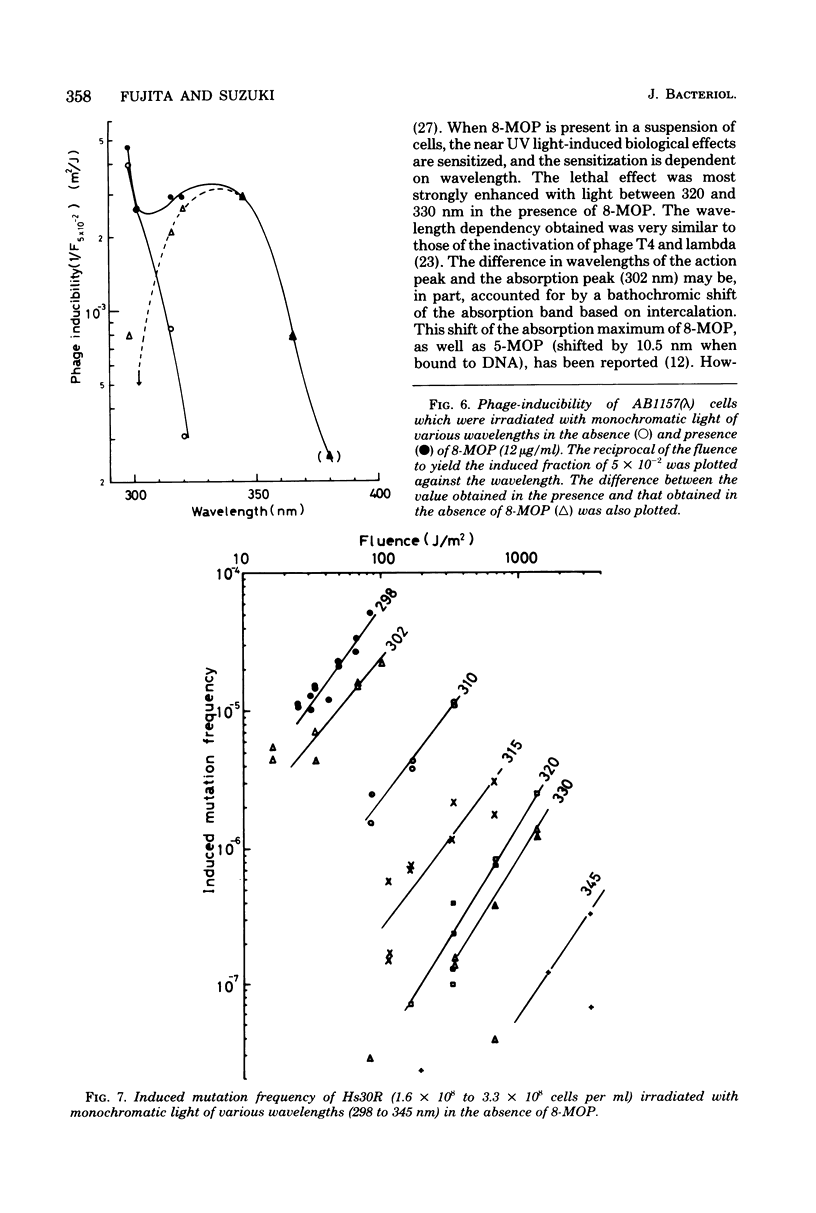
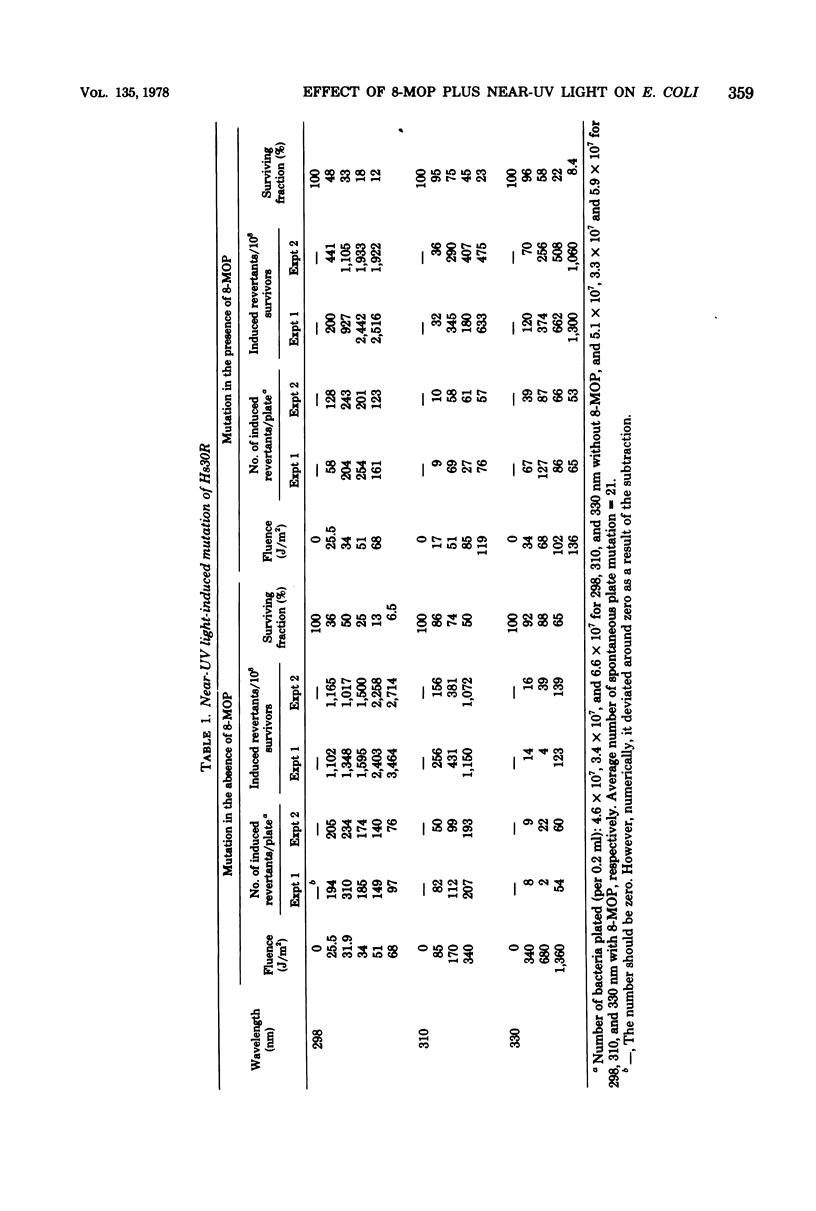
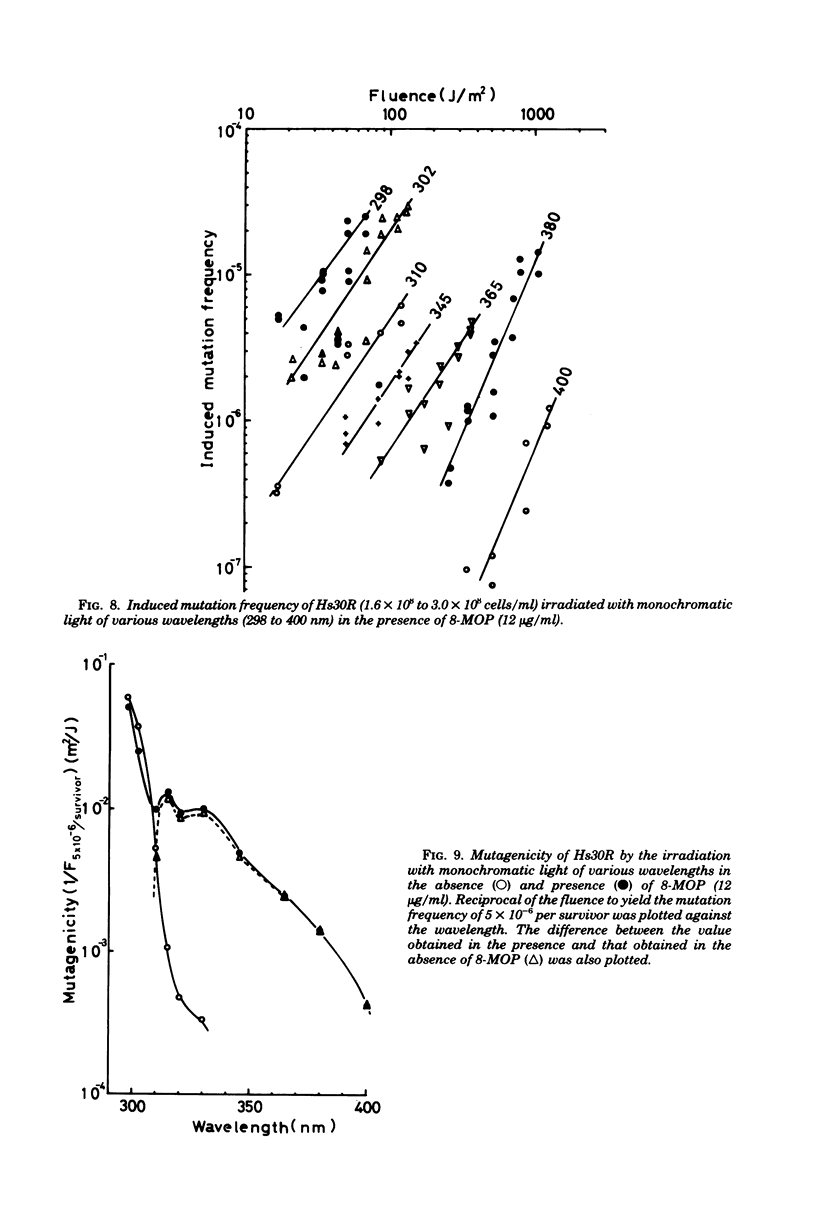
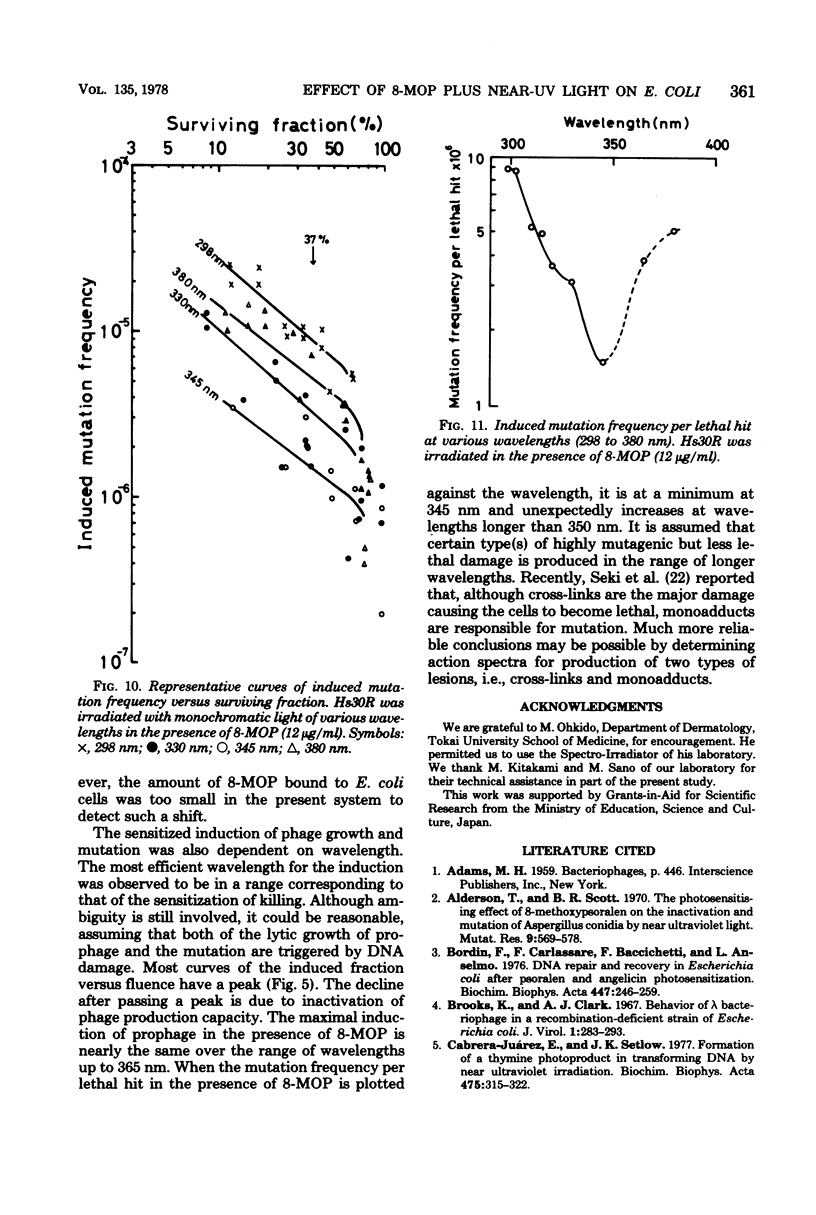
Wavelength dependency of photo-inactivation and photoinduced reverse mutation of Escherichia coli sensitized with 8-methoxypsoralen, and wavelength dependency of photoinduction of lambda prophage from the sensitized lysogen were measured in a range of 298 to 400 nm. The most efficient sensitization for these biological effects was observed between 320 and 340 nm. In the presence of 8-methyoxypsoralen, the induced mutation frequency per lethal hit was highest of 298 nm in the range examined and was gradually decreased with increasing wavelength to a minimum frequency at 345 nm. This finding may be a reflection of the production of more than one type of lesions which have different efficiencies for mutation compared with the killing efficiency.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderson T., Scott B. R. The photosensitising effect of 8-methoxypsoralen on the inactivation and mutation of Aspergillus conidia by near ultraviolet light. Mutat Res. 1970 Jun;9(6):569–578. doi: 10.1016/0027-5107(70)90102-8. [DOI] [PubMed] [Google Scholar]
- Bordin F., Carlassare F., Baccichetti F., Anselmo L. DNA repair and recovery in Escherichia coli after psoralen and angelicin photosensitization. Biochim Biophys Acta. 1976 Oct 18;447(3):249–259. doi: 10.1016/0005-2787(76)90048-4. [DOI] [PubMed] [Google Scholar]
- Brooks K., Clark A. J. Behavior of lambda bacteriophage in a recombination deficienct strain of Escherichia coli. J Virol. 1967 Apr;1(2):283–293. doi: 10.1128/jvi.1.2.283-293.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbera-Juárez E., Setlow J. K. Formation of a thymine photoproduct in transforming DNA by near ultraviolet irradiation. Biochim Biophys Acta. 1977 Mar 18;475(2):315–322. doi: 10.1016/0005-2787(77)90022-3. [DOI] [PubMed] [Google Scholar]
- Cole R. S. Inactivation of Escherichia coli, F' episomes at transfer, and bacteriophage lambda by psoralen plus 360-nm light: significance of deoxyribonucleic acid cross-links. J Bacteriol. 1971 Sep;107(3):846–852. doi: 10.1128/jb.107.3.846-852.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. S., Levitan D., Sinden R. R. Removal of psoralen interstrand cross-links from DNA of Escherichia coli: mechanism and genetic control. J Mol Biol. 1976 May 5;103(1):39–59. doi: 10.1016/0022-2836(76)90051-6. [DOI] [PubMed] [Google Scholar]
- Cole R. S. Light-induced cross-linking of DNA in the presence of a furocoumarin (psoralen). Studies with phage lambda, Escherichia coli, and mouse leukemia cells. Biochim Biophys Acta. 1970 Sep 17;217(1):30–39. doi: 10.1016/0005-2787(70)90119-x. [DOI] [PubMed] [Google Scholar]
- Cole R. S. Psoralen monoadducts and interstrand cross-links in DNA. Biochim Biophys Acta. 1971 Nov 29;254(1):30–39. doi: 10.1016/0005-2787(71)90111-0. [DOI] [PubMed] [Google Scholar]
- Dall'Acqua F., Marciani S., Rodighiero G. Photoreactivity (3655 Angstrom) of various skin-photosensitizing furocoumarins with nucleic acids. Z Naturforsch B. 1969 Mar;24(3):307–314. doi: 10.1515/znb-1969-0309. [DOI] [PubMed] [Google Scholar]
- Dall'Acqua F., Marciani S., Rodighiero G. The action spectrum of xanthotoxin and bergapten for the photoreaction with native DNA. Z Naturforsch B. 1969 Jun;24(6):667–671. [PubMed] [Google Scholar]
- Drake J. W., McGuire J. Properties of r mutants of bacteriophage T4 photodynamically induced in the presence of thiopyronin and psoralen. J Virol. 1967 Apr;1(2):260–267. doi: 10.1128/jvi.1.2.260-267.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., SIMSON E., THERIOT L. A LOCUS THAT CONTROLS FILAMENT FORMATION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI K-12. Genetics. 1964 Feb;49:237–246. doi: 10.1093/genetics/49.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igali S., Bridges B. A., Ashwood-Smith M. J., Scott B. R. Mutagenesis in Escherichia coli. IV. Photosensitization to near ultraviolet light by 8-methoxypsoralen. Mutat Res. 1970 Jan;9(1):21–30. doi: 10.1016/0027-5107(70)90067-9. [DOI] [PubMed] [Google Scholar]
- Kondo S., Ichikawa H., Iwo K., Kato T. Base-change mutagenesis and prophage induction in strains of Escherichia coli with different DNA repair capacities. Genetics. 1970 Oct;66(2):187–217. doi: 10.1093/genetics/66.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S., Kato T. Action spectra for photoreactivation of killing and mutation to prototrophy in U.V.-sensitive strains of Escherichia Coli possessing and lacking photoreactivating enzyme. Photochem Photobiol. 1966 Nov-Dec;5(11):827–837. doi: 10.1111/j.1751-1097.1966.tb05929.x. [DOI] [PubMed] [Google Scholar]
- LWOFF A. Lysogeny. Bacteriol Rev. 1953 Dec;17(4):269–337. doi: 10.1128/br.17.4.269-337.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott B. R., Alderson T. The random (non-specific) forward mutational response of gene loci in Aspergillus conidia after photosensitisation to near ultraviolet light (365 nm) by 8-methoxypsoralen. Mutat Res. 1971 May;12(1):29–34. doi: 10.1016/0027-5107(71)90069-8. [DOI] [PubMed] [Google Scholar]
- Seki T., Nozu K., Kondo S. Differential causes of mutation and killing in Escherichia coli after psoralen plus light treatment: monoadducts and cross-links. Photochem Photobiol. 1978 Jan;27(1):19–24. doi: 10.1111/j.1751-1097.1978.tb07559.x. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Fujita H., Yanagisawa F., Kitakami M. Action spectrum for the photo-inactivation of lambda and T-4 bacteriophages sensitized with 8-methoxypsoralen. Photochem Photobiol. 1977 Jul;26(1):49–51. doi: 10.1111/j.1751-1097.1977.tb07447.x. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Yanagisawa F., Fujita H., Ohkido M. Inactivation of T4 bacteriophage by near ultraviolet light in the presence of 8-methoxypsoralen. J Dermatol. 1978 Feb;5(1):15–19. doi: 10.1111/j.1346-8138.1978.tb01043.x. [DOI] [PubMed] [Google Scholar]
- Swanbeck G., Thyresson M. Induction of respiration-deficient mutants in yeast by psoralen and light. J Invest Dermatol. 1974 Aug;63(2):242–244. doi: 10.1111/1523-1747.ep12680024. [DOI] [PubMed] [Google Scholar]
- Tyrrell R. M. Induction of pyrimidine dimers in bacterial DNA by 365 nm radiation. Photochem Photobiol. 1973 Jan;17(1):69–73. doi: 10.1111/j.1751-1097.1973.tb06334.x. [DOI] [PubMed] [Google Scholar]
- Tyrrell R. M., Ley R. D., Webb R. B. Induction of single-strand breaks (alkali-labile bonds) in bacterial and phage DNA by near UV (365 nm) radiation. Photochem Photobiol. 1974 Nov;20(5):395–398. doi: 10.1111/j.1751-1097.1974.tb06593.x. [DOI] [PubMed] [Google Scholar]
- WITKIN E. M. PHOTOREVERSAL AND "DARK REPAIR" OF MUTATIONS TO PROTOTROPHY INDUCED BY ULTRAVIOLET LIGHT IN PHOTOREACTIVABLE AND NON-PHOTOREACTIVABLE STRAINS OF ESCHERICHIA COLI. Mutat Res. 1964 May;106:22–36. doi: 10.1016/0027-5107(64)90049-1. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


