Abstract
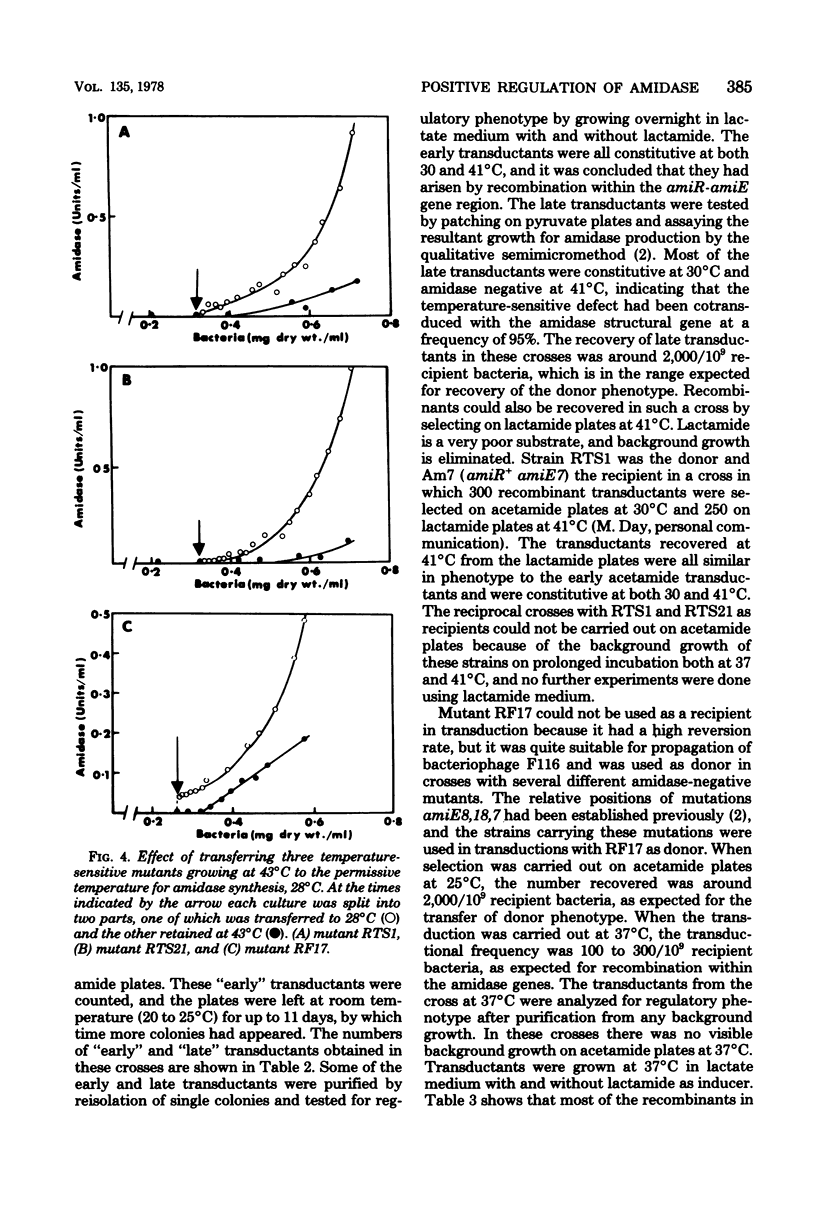
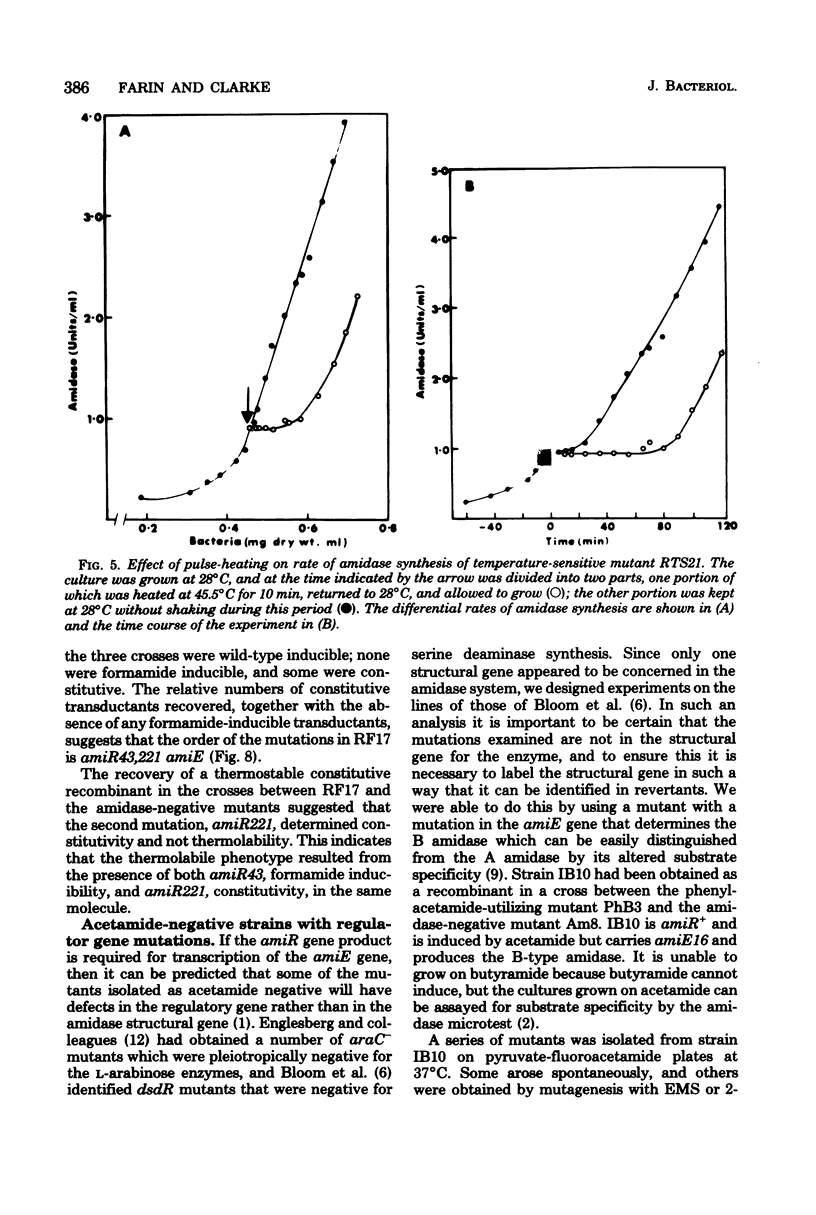
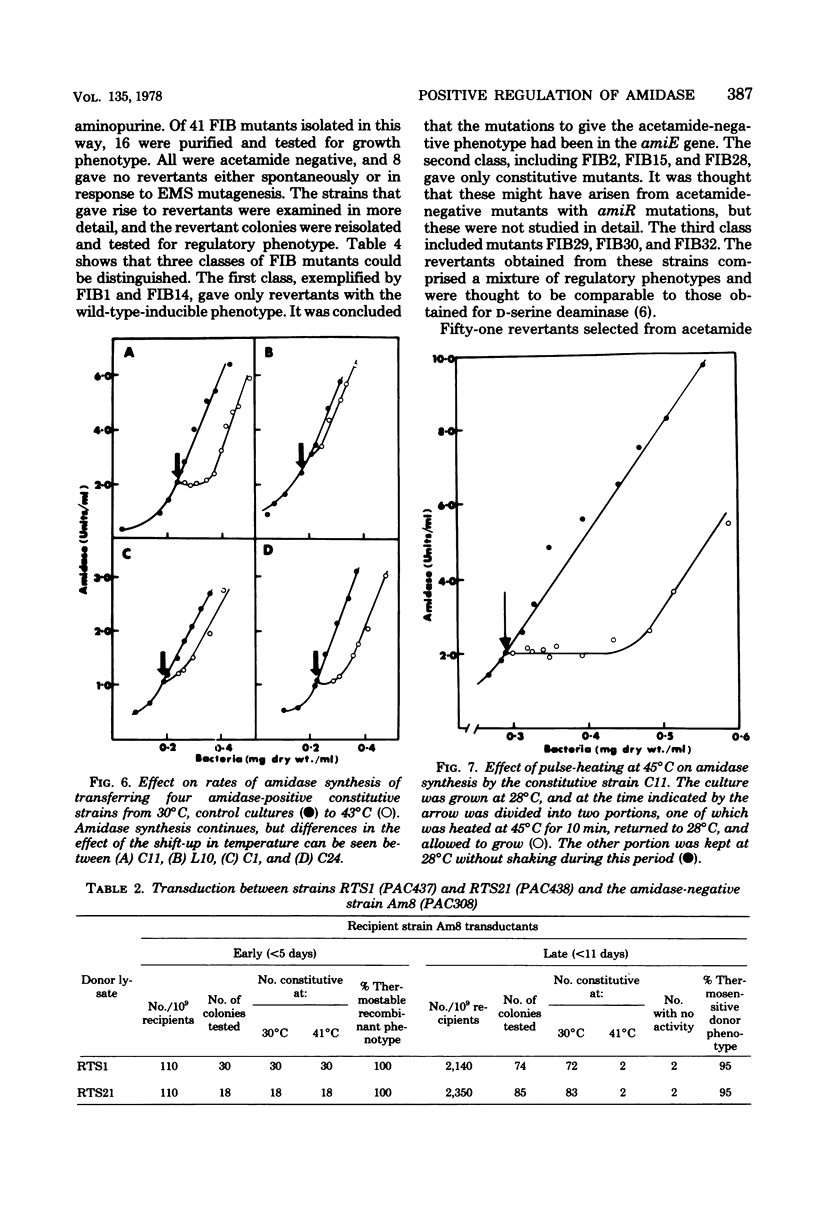
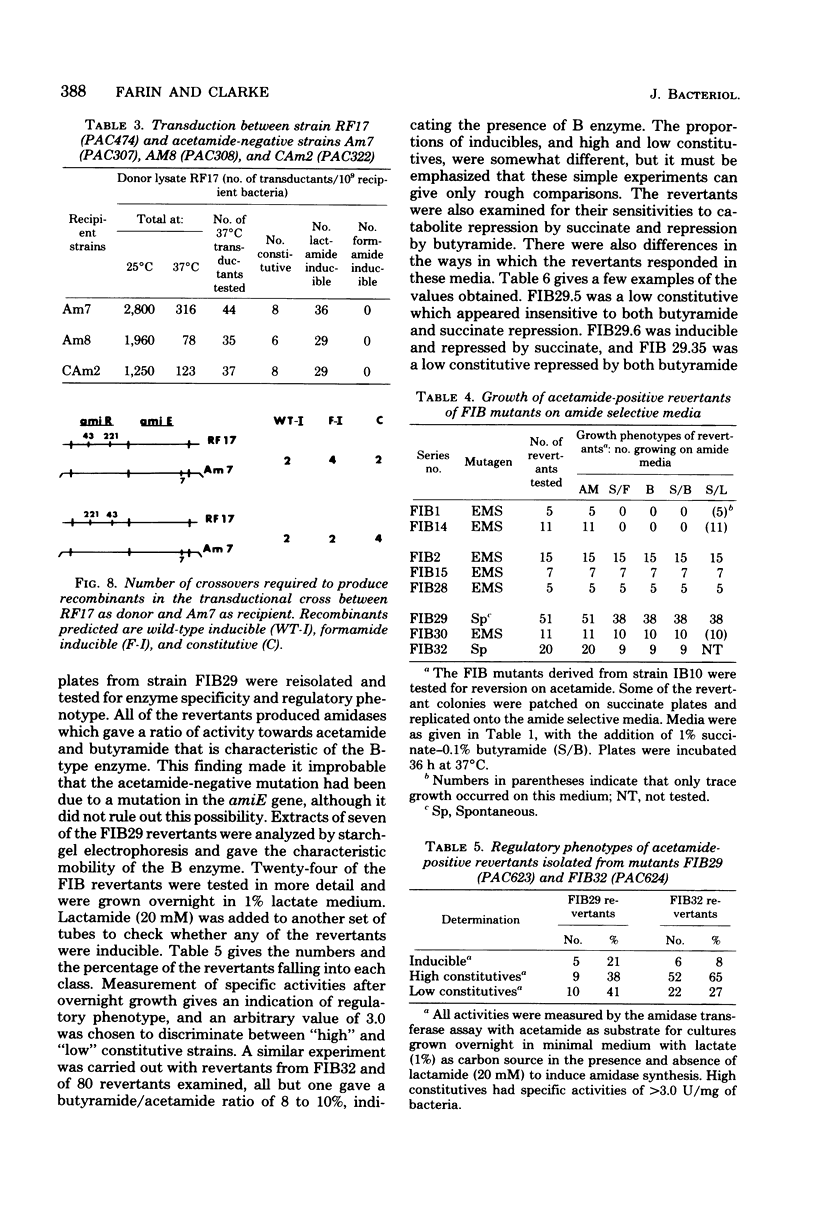
Mutants of Pseudomonas aeruginosa were isolated that were acetamide-negative in growth phenotype at 41 degrees C and constitutive for amidase synthesis at 28 degrees C. Two mutants were derived from the magno-constitutive amidase mutant PAC111 (C11), and a third from a mutant that had enhanced inducibility by formamide, PAC153 (F6). The three temperature-sensitive mutants produced amidases with the same thermal stabilities as the wild-type enzyme. Cultures growing exponentially at 28 degrees C, synthesizing amidase constitutively, ceased amidase synthesis almost immediately on transfer to 41 degrees C. Cultures growing at 41 degrees C were transferred to 28 degrees C and had a lag of about 0.5 of a generation before amidase synthesis became detectable. Pulse-heating for 10 min at 45 degrees C of a culture growing exponentially at 28 degrees C resulted in a lag of about 0.5 of a generation before amidase synthesis recommenced after returning to 28 degrees C. Acetamide-negative mutants that were unable to synthesize amidase at any growth temperature were isolated from an inducible strain producing the mutant B amidase PAC398 (IB10). Two mutants were examined that gave revertants producing B amidase but with novel regulatory phenotypes. It is suggested that amidase synthesis is regulated by positive control exerted by gene amiR.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAMMAR W. J., CLARKE P. H. INDUCTION AND REPRESSION OF PSEUDOMONAS AERUGINOSA AMIDASE. J Gen Microbiol. 1964 Dec;37:307–319. doi: 10.1099/00221287-37-3-307. [DOI] [PubMed] [Google Scholar]
- Beckwith J., Rossow P. Analysis of genetic regulatory mechanisms. Annu Rev Genet. 1974;8:1–13. doi: 10.1146/annurev.ge.08.120174.000245. [DOI] [PubMed] [Google Scholar]
- Betz J. L., Brown J. E., Clarke P. H., Day M. Genetic analysis of amidase mutants of Pseudomonas aeruginosa. Genet Res. 1974 Jun;23(3):335–359. doi: 10.1017/s001667230001497x. [DOI] [PubMed] [Google Scholar]
- Betz J. L., Clarke P. H. Selective evolution of phenylacetamide-utilizing strains of Pseudomonas aeruginosa. J Gen Microbiol. 1972 Nov;73(1):161–174. doi: 10.1099/00221287-73-1-161. [DOI] [PubMed] [Google Scholar]
- Bloom F. R. Isolation and characterization of catabolite-resistant mutants in the D-serine deaminase system of Escherichia coli K-12. J Bacteriol. 1975 Mar;121(3):1085–1091. doi: 10.1128/jb.121.3.1085-1091.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom F. R., McFall E. Isolation and characterization of D-serine deaminase constitutive mutants by utilization of D-serine as sole carbon or nitrogen source. J Bacteriol. 1975 Mar;121(3):1078–1084. doi: 10.1128/jb.121.3.1078-1084.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom F. R., McFall E., Young M. C., Carothers A. M. Positive control in the D-serine deaminase system of Escherichia coli K-12. J Bacteriol. 1975 Mar;121(3):1092–1101. doi: 10.1128/jb.121.3.1092-1101.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brammar W. J., Clarke P. H., Skinner A. J. Biochemical and genetic studies with regulator mutants of the Pseudomonas aeruginosa 8602 amidase system. J Gen Microbiol. 1967 Apr;47(1):87–102. doi: 10.1099/00221287-47-1-87. [DOI] [PubMed] [Google Scholar]
- Brown J. E., Brown P. R., Clarke P. H. Butyramide-utilizing mutants of Pseudomonas aeruginosa 8602 which produce an amidase with altered substrate specificity. J Gen Microbiol. 1969 Aug;57(2):273–285. doi: 10.1099/00221287-57-2-273. [DOI] [PubMed] [Google Scholar]
- Brown J. E., Clarke P. H. Mutations in a regulator gene allowing Pseudomonas aeruginosa 8602 to grow on butyramide. J Gen Microbiol. 1970 Dec;64(3):329–342. doi: 10.1099/00221287-64-3-329. [DOI] [PubMed] [Google Scholar]
- Englesberg E., Irr J., Power J., Lee N. Positive control of enzyme synthesis by gene C in the L-arabinose system. J Bacteriol. 1965 Oct;90(4):946–957. doi: 10.1128/jb.90.4.946-957.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englesberg E., Wilcox G. Regulation: positive control. Annu Rev Genet. 1974;8:219–242. doi: 10.1146/annurev.ge.08.120174.001251. [DOI] [PubMed] [Google Scholar]
- HOLLOWAY B. W., EGAN J. B., MONK M. Lysogeny in Pseudomonas aeruginosa. Aust J Exp Biol Med Sci. 1960 Aug;38:321–329. doi: 10.1038/icb.1960.34. [DOI] [PubMed] [Google Scholar]
- Heffernan L., Bass R., Englesberg E. Mutations affecting catabolite repression of the L-arabinose regulon in Escherichia coli B/r. J Bacteriol. 1976 Jun;126(3):1119–1131. doi: 10.1128/jb.126.3.1119-1131.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irr J., Englesberg E. Control of expression of the L-arabinose operon in temperature-sensitive mutants of gene araC in Escherichia coli B-r. J Bacteriol. 1971 Jan;105(1):136–141. doi: 10.1128/jb.105.1.136-141.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLY M., CLARKE P. H. An inducible amidase produced by a strain of Pseudomonas aeruginosa. J Gen Microbiol. 1962 Feb;27:305–316. doi: 10.1099/00221287-27-2-305. [DOI] [PubMed] [Google Scholar]
- McFall E. Role of adenosine 3',5'-cyclic monophosphate and its specific binding protein in the regulation of D-serine deaminase synthesis. J Bacteriol. 1973 Feb;113(2):781–785. doi: 10.1128/jb.113.2.781-785.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SADLER J. R., NOVICK A. THE PROPERTIES OF REPRESSOR AND THE KINETICS OF ITS ACTION. J Mol Biol. 1965 Jun;12:305–327. doi: 10.1016/s0022-2836(65)80255-8. [DOI] [PubMed] [Google Scholar]
- Smyth P. F., Clarke P. H. Catabolite repression of Pseudomonas aeruginosa amidase: isolation of promotor mutants. J Gen Microbiol. 1975 Sep;90(1):91–99. doi: 10.1099/00221287-90-1-91. [DOI] [PubMed] [Google Scholar]
- Smyth P. F., Clarke P. H. Catabolite repression of Pseudomonas aeruginosa amidase: the effect of carbon source on amidase synthesis. J Gen Microbiol. 1975 Sep;90(1):81–90. doi: 10.1099/00221287-90-1-81. [DOI] [PubMed] [Google Scholar]
- Wheelis L. The genetics of dissimilarity pathways in Pseudomonas. Annu Rev Microbiol. 1975;29:505–524. doi: 10.1146/annurev.mi.29.100175.002445. [DOI] [PubMed] [Google Scholar]
- Worsey M. J., Franklin F. C., Williams P. A. Regulation of the degradative pathway enzymes coded for by the TOL plasmid (pWWO) from Pseudomonas putida mt-2. J Bacteriol. 1978 Jun;134(3):757–764. doi: 10.1128/jb.134.3.757-764.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsey M. J., Williams P. A. Characterization of a spontaneously occurring mutant of the TOL20 plasmid in Pseudomonas putida MT20: possible regulatory implications. J Bacteriol. 1977 Jun;130(3):1149–1158. doi: 10.1128/jb.130.3.1149-1158.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


