Abstract
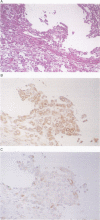
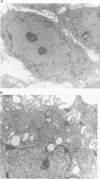
Bladder cancer cells have been shown to secrete a variety of factors that are not related to cells of urothelial origin. The histogenesis of these tumour developments is uncertain, and a variety of theories have been previously reported. In the present manuscript, we identify the factors constitutively produced by a human bladder cancer cell line (KU-19-19) that was found to produce beta human chorionic gonadotrophin (beta-hCG), granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin 1alpha (IL-1alpha), interleukin 6 (IL-6) and interleukin 8 (IL-8). The cells were obtained from a case of metastatic carcinoma that was originally diagnosed to be a grade 3 (WHO classification), invasive transitional cell carcinoma of the bladder. On microscopic observation, the cultured cells exhibited an epithelial appearance with vacuole formation in their cytoplasm. Ultrastructural observations revealed relatively marked microvilli and a tight junction. Significant amounts of beta-hCG, G-CSF, GM-CSF, IL-1alpha, IL-6 and IL-8 concentrations in the supernatant from cultured cells were demonstrated by enzyme-linked immunosorbent assays, while the expression of mRNA of these marker proteins in cancer cells was also significantly exhibited by reverse transcription polymerase chain reaction (RT-PCR). In addition, the expression of G-CSF receptor and IL-6 receptor mRNA was also shown by RT-PCR. Xenograft transplantability using nude mice was observed in association with the presence of severe neutrophilia in the peripheral blood. These results indicate that this cell line appears to be an effective model for the study of transitional cell carcinoma of the bladder with multipotent differentiation potentials.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin G. C., Gasson J. C., Kaufman S. E., Quan S. G., Williams R. E., Avalos B. R., Gazdar A. F., Golde D. W., DiPersio J. F. Nonhematopoietic tumor cells express functional GM-CSF receptors. Blood. 1989 Mar;73(4):1033–1037. [PubMed] [Google Scholar]
- Benham F., Cottell D. C., Franks L. M., Wilson P. D. Alkaline phosphatase activity in human bladder tumor cell lines. J Histochem Cytochem. 1977 Apr;25(4):266–274. doi: 10.1177/25.4.870558. [DOI] [PubMed] [Google Scholar]
- Berdel W. E., Danhauser-Riedl S., Steinhauser G., Winton E. F. Various human hematopoietic growth factors (interleukin-3, GM-CSF, G-CSF) stimulate clonal growth of nonhematopoietic tumor cells. Blood. 1989 Jan;73(1):80–83. [PubMed] [Google Scholar]
- Chodak G. W., Summerhayes I. Detection of angiogenesis activity in malignant bladder tissue and cells. J Urol. 1984 Nov;132(5):1032–1035. doi: 10.1016/s0022-5347(17)49993-9. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Crawford S. M., Ledermann J. A., Turkie W., Rustin G. J., Begent R. H., Newlands E. S., Bagshawe K. D. Is ectopic production of human chorionic gonadotrophin (hCG) or alpha fetoprotein (AFP) by tumours a marker of chemosensitivity? Eur J Cancer Clin Oncol. 1986 Dec;22(12):1483–1487. doi: 10.1016/0277-5379(86)90084-2. [DOI] [PubMed] [Google Scholar]
- Dedhar S., Gaboury L., Galloway P., Eaves C. Human granulocyte-macrophage colony-stimulating factor is a growth factor active on a variety of cell types of nonhemopoietic origin. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9253–9257. doi: 10.1073/pnas.85.23.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetri G. D., Griffin J. D. Granulocyte colony-stimulating factor and its receptor. Blood. 1991 Dec 1;78(11):2791–2808. [PubMed] [Google Scholar]
- Dexeus F., Logothetis C., Hossan E., Samuels M. L. Carcinoembryonic antigen and beta-human chorionic gonadotropin as serum markers for advanced urothelial malignancies. J Urol. 1986 Aug;136(2):403–407. doi: 10.1016/s0022-5347(17)44882-8. [DOI] [PubMed] [Google Scholar]
- Droller M. J., Perlmann P., Schneider M. U. Enhancement of natural and antibody-dependent lymphocyte cytotoxicity by drugs which inhibit prostaglandin production by tumor target cells. Cell Immunol. 1978 Aug;39(1):154–164. doi: 10.1016/0008-8749(78)90090-4. [DOI] [PubMed] [Google Scholar]
- Gasson J. C., Kaufman S. E., Weisbart R. H., Tomonaga M., Golde D. W. High-affinity binding of granulocyte-macrophage colony-stimulating factor to normal and leukemic human myeloid cells. Proc Natl Acad Sci U S A. 1986 Feb;83(3):669–673. doi: 10.1073/pnas.83.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammatico D., Grignon D. J., Eberwein P., Shepherd R. R., Hearn S. A., Walton J. C. Transitional cell carcinoma of the renal pelvis with choriocarcinomatous differentiation. Immunohistochemical and immunoelectron microscopic assessment of human chorionic gonadotropin production by transitional cell carcinoma of the urinary bladder. Cancer. 1993 Mar 1;71(5):1835–1841. doi: 10.1002/1097-0142(19930301)71:5<1835::aid-cncr2820710519>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Griffin J. D., Young D., Herrmann F., Wiper D., Wagner K., Sabbath K. D. Effects of recombinant human GM-CSF on proliferation of clonogenic cells in acute myeloblastic leukemia. Blood. 1986 May;67(5):1448–1453. [PubMed] [Google Scholar]
- Hall R. R., Laurence D. J., Neville A. M., Wallace D. M. Carcinoembryonic antigen and urothelial carcinoma. Br J Urol. 1973 Feb;45(1):88–92. [PubMed] [Google Scholar]
- Iles R. K., Chard T. Human chorionic gonadotropin expression by bladder cancers: biology and clinical potential. J Urol. 1991 Mar;145(3):453–458. doi: 10.1016/s0022-5347(17)38367-2. [DOI] [PubMed] [Google Scholar]
- Kaashoek J. G., Mout R., Falkenburg J. H., Willemze R., Fibbe W. E., Landegent J. E. Cytokine production by the bladder carcinoma cell line 5637: rapid analysis of mRNA expression levels using a cDNA-PCR procedure. Lymphokine Cytokine Res. 1991 Jun;10(3):231–235. [PubMed] [Google Scholar]
- Kawano M., Hirano T., Matsuda T., Taga T., Horii Y., Iwato K., Asaoku H., Tang B., Tanabe O., Tanaka H. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature. 1988 Mar 3;332(6159):83–85. doi: 10.1038/332083a0. [DOI] [PubMed] [Google Scholar]
- Kelleher C., Miyauchi J., Wong G., Clark S., Minden M. D., McCulloch E. A. Synergism between recombinant growth factors, GM-CSF and G-CSF, acting on the blast cells of acute myeloblastic leukemia. Blood. 1987 May;69(5):1498–1503. [PubMed] [Google Scholar]
- Kinjo M., Oka K., Naito S., Kohga S., Tanaka K., Oboshi S., Hayata Y., Yasumoto K. Thromboplastic and fibrinolytic activities of cultured human cancer cell lines. Br J Cancer. 1979 Jan;39(1):15–23. doi: 10.1038/bjc.1979.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing E. M., Hanson P., Ulrich P., Erturk E. Epidermal growth factor--interactions with normal and malignant urothelium: in vivo and in situ studies. J Urol. 1987 Nov;138(5):1329–1335. doi: 10.1016/s0022-5347(17)43593-2. [DOI] [PubMed] [Google Scholar]
- Miki S., Iwano M., Miki Y., Yamamoto M., Tang B., Yokokawa K., Sonoda T., Hirano T., Kishimoto T. Interleukin-6 (IL-6) functions as an in vitro autocrine growth factor in renal cell carcinomas. FEBS Lett. 1989 Jul 3;250(2):607–610. doi: 10.1016/0014-5793(89)80805-1. [DOI] [PubMed] [Google Scholar]
- Neal D. E., Smith K., Fennelly J. A., Bennett M. K., Hall R. R., Harris A. L. Epidermal growth factor receptor in human bladder cancer: a comparison of immunohistochemistry and ligand binding. J Urol. 1989 Mar;141(3):517–521. doi: 10.1016/s0022-5347(17)40877-9. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Cancer progression and growth: relationship of paracrine and autocrine growth mechanisms to organ preference of metastasis. Exp Cell Res. 1993 Feb;204(2):171–180. doi: 10.1006/excr.1993.1022. [DOI] [PubMed] [Google Scholar]
- Oster W., Cicco N. A., Klein H., Hirano T., Kishimoto T., Lindemann A., Mertelsmann R. H., Herrmann F. Participation of the cytokines interleukin 6, tumor necrosis factor-alpha, and interleukin 1-beta secreted by acute myelogenous leukemia blasts in autocrine and paracrine leukemia growth control. J Clin Invest. 1989 Aug;84(2):451–457. doi: 10.1172/JCI114186. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Park L. S., Friend D., Gillis S., Urdal D. L. Characterization of the cell surface receptor for human granulocyte/macrophage colony-stimulating factor. J Exp Med. 1986 Jul 1;164(1):251–262. doi: 10.1084/jem.164.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe J. M., Rapoport A. P. Hemopoietic growth factors: a review. J Clin Pharmacol. 1992 Jun;32(6):486–501. doi: 10.1177/009127009203200602. [DOI] [PubMed] [Google Scholar]
- Russell P. J., Wills E. J., Philips J., Wass J., Jelbart M., Gregory P., Raghavan D. Features of squamous and adenocarcinoma in the same cell in a xenografted human transitional cell carcinoma: evidence of a common histogenesis? Urol Res. 1988;16(2):79–84. doi: 10.1007/BF00261960. [DOI] [PubMed] [Google Scholar]
- Sato K., Terada K., Sugiyama T., Sugiyama T., Masuda H., Kakinuma H., Kato T. Granulocyte colony-stimulating factor produced by bladder carcinoma of a patient with leukemoid reaction did not affect proliferation of the tumor cells. J Urol. 1994 Jun;151(6):1687–1690. doi: 10.1016/s0022-5347(17)35345-4. [DOI] [PubMed] [Google Scholar]
- Serve H., Steinhauser G., Oberberg D., Flegel W. A., Northoff H., Berdel W. E. Studies on the interaction between interleukin 6 and human malignant nonhematopoietic cell lines. Cancer Res. 1991 Aug 1;51(15):3862–3866. [PubMed] [Google Scholar]
- Shimamura K., Fujimoto J., Hata J., Akatsuka A., Ueyama Y., Watanabe T., Tamaoki N. Establishment of specific monoclonal antibodies against recombinant human granulocyte colony-stimulating factor (hG-CSF) and their application for immunoperoxidase staining of paraffin-embedded sections. J Histochem Cytochem. 1990 Feb;38(2):283–286. doi: 10.1177/38.2.1688901. [DOI] [PubMed] [Google Scholar]
- Sires C., Neely S., Skinner D. Leukemoid reaction in a patient with bladder and prostatic cancer. J Urol. 1986 Feb;135(2):366–367. doi: 10.1016/s0022-5347(17)45641-2. [DOI] [PubMed] [Google Scholar]
- Smith K., Fennelly J. A., Neal D. E., Hall R. R., Harris A. L. Characterization and quantitation of the epidermal growth factor receptor in invasive and superficial bladder tumors. Cancer Res. 1989 Nov 1;49(21):5810–5815. [PubMed] [Google Scholar]
- Souza L. M., Boone T. C., Gabrilove J., Lai P. H., Zsebo K. M., Murdock D. C., Chazin V. R., Bruszewski J., Lu H., Chen K. K. Recombinant human granulocyte colony-stimulating factor: effects on normal and leukemic myeloid cells. Science. 1986 Apr 4;232(4746):61–65. doi: 10.1126/science.2420009. [DOI] [PubMed] [Google Scholar]
- Tachibana M. Studies on cellular adhesiveness in five different culture cell lines derived from carcinoma of the urinary bladder. Keio J Med. 1982 Oct;31(3):127–148. doi: 10.2302/kjm.31.127. [DOI] [PubMed] [Google Scholar]
- Tomonaga M., Golde D. W., Gasson J. C. Biosynthetic (recombinant) human granulocyte-macrophage colony-stimulating factor: effect on normal bone marrow and leukemia cell lines. Blood. 1986 Jan;67(1):31–36. [PubMed] [Google Scholar]
- UKCCCR guidelines for the welfare of animals in experimental neoplasia. Br J Cancer. 1988 Jul;58(1):109–113. doi: 10.1038/bjc.1988.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellenga E., Young D. C., Wagner K., Wiper D., Ostapovicz D., Griffin J. D. The effects of GM-CSF and G-CSF in promoting growth of clonogenic cells in acute myeloblastic leukemia. Blood. 1987 Jun;69(6):1771–1776. [PubMed] [Google Scholar]
- Welte K., Platzer E., Lu L., Gabrilove J. L., Levi E., Mertelsmann R., Moore M. A. Purification and biochemical characterization of human pluripotent hematopoietic colony-stimulating factor. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1526–1530. doi: 10.1073/pnas.82.5.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzler M., Estrov Z., Talpaz M., Markowitz A., Gutterman J. U., Kurzrock R. Granulocyte-macrophage colony-stimulating factor as a cause of paraneoplastic leukaemoid reaction in advanced transitional cell carcinoma. J Intern Med. 1993 Oct;234(4):417–420. doi: 10.1111/j.1365-2796.1993.tb00765.x. [DOI] [PubMed] [Google Scholar]
- Young D. C., Griffin J. D. Autocrine secretion of GM-CSF in acute myeloblastic leukemia. Blood. 1986 Nov;68(5):1178–1181. [PubMed] [Google Scholar]
- Zinzar S. N., Svet-Moldavsky G. J., Fogh J., Mann P. E., Arlin Z., Iliescu K., Holland J. F. Elaboration of granulocyte-macrophage colony-stimulating factor by human tumor cell lines and normal urothelium. Exp Hematol. 1985 Jul;13(6):574–580. [PubMed] [Google Scholar]