Abstract
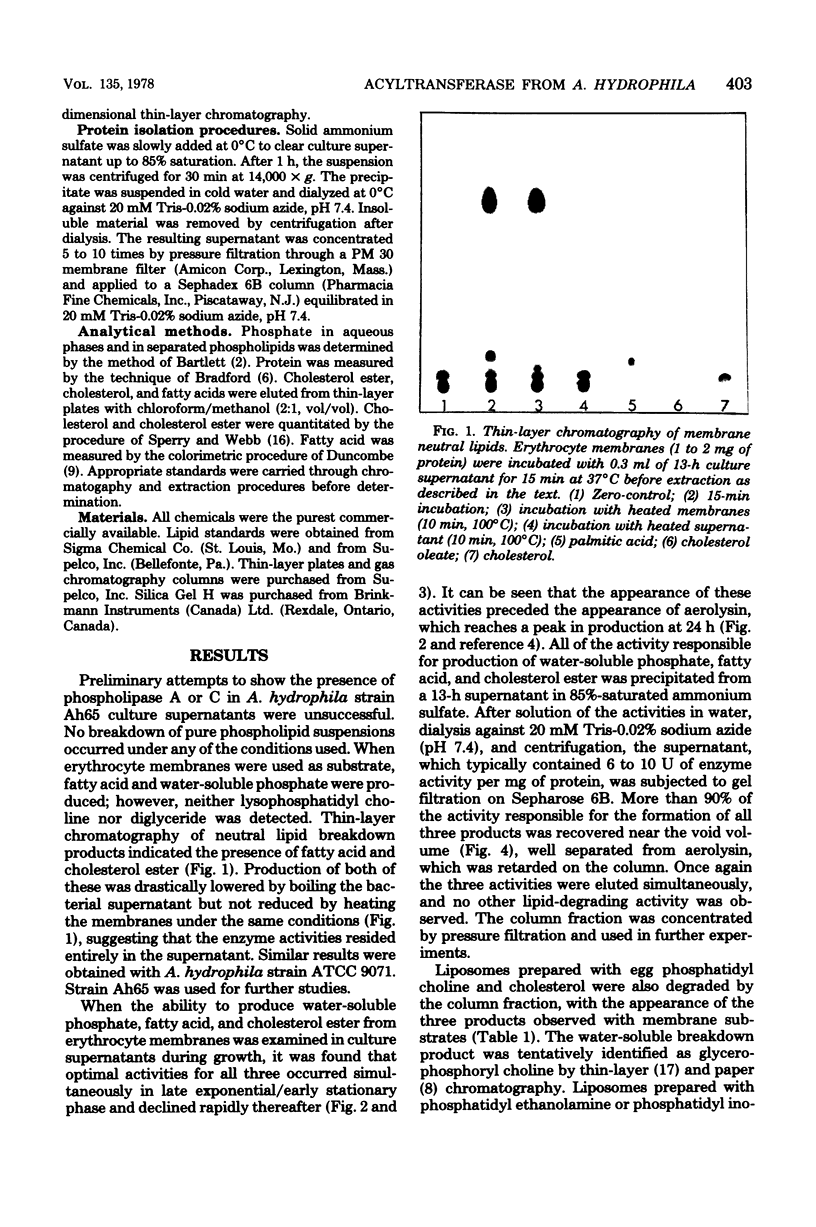
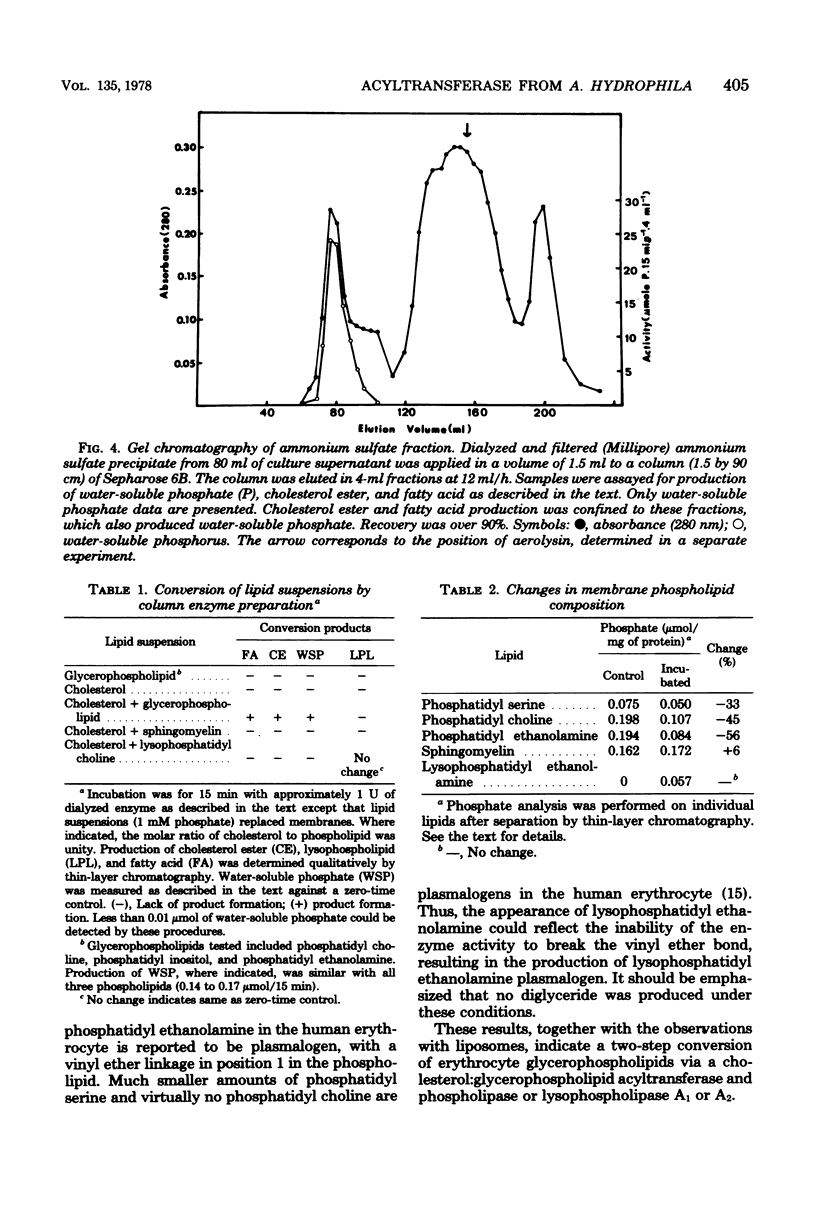
Human erythrocyte membrane glycerophospholipids are deacylated by Aeromonas hydrophila 13-h culture supernatants, resulting in the production of cholesterol ester, free fatty acid, and water-soluble phosphates. This activity appears to be due to the actions of an acyltransferase (phosphatide:cholesterol acyltransferase, EC 2.3.1 group) and a phospholipase (phosphatide acyl-hydrolase). The enzyme activities are produced simultaneously in late exponential/early stationary phase, are precipitated together from the culture supernatant with 85% ammonium sulfate, and are eluted together near the void volume during gel filtration on Sepharose 6B. These results suggest that A. hydrophila produces a multienzyme complex with an unusual mode of action on membrane lipids. The complex is distinct from the hemolytic factor aerolysin, which is also produced by A. hydrophila.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Bernheimer A. W., Avigad L. S., Avigad G. Interactions between aerolysin, erythrocytes, and erythrocyte membranes. Infect Immun. 1975 Jun;11(6):1312–1319. doi: 10.1128/iai.11.6.1312-1319.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer A. W., Avigad L. S. Partial characterization of aerolysin, a lytic exotoxin from Aeromonas hydrophila. Infect Immun. 1974 Jun;9(6):1016–1021. doi: 10.1128/iai.9.6.1016-1021.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Buckley J. T., Hawthorne J. N. Erythrocyte membrane polyphosphoinositide metabolism and the regulation of calcium binding. J Biol Chem. 1972 Nov 25;247(22):7218–7223. [PubMed] [Google Scholar]
- DAWSON R. M. A hydrolytic procedure for the identification and estimation of individual phospholipids in biological samples. Biochem J. 1960 Apr;75:45–53. doi: 10.1042/bj0750045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncombe W. G. The colorimetric micro-determination of long-chain fatty acids. Biochem J. 1963 Jul;88(1):7–10. doi: 10.1042/bj0880007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatt S., Barenholz Y. Enzymes of complex lipid metabolism. Annu Rev Biochem. 1973;42(0):61–90. doi: 10.1146/annurev.bi.42.070173.000425. [DOI] [PubMed] [Google Scholar]
- Glenn A. R. Production of extracellular proteins by bacteria. Annu Rev Microbiol. 1976;30:41–62. doi: 10.1146/annurev.mi.30.100176.000353. [DOI] [PubMed] [Google Scholar]
- Owens J. J. The egg yolk reaction produced by several species of bacteria. J Appl Bacteriol. 1974 Mar;37(1):137–148. doi: 10.1111/j.1365-2672.1974.tb00424.x. [DOI] [PubMed] [Google Scholar]
- SPERRY W. M., WEBB M. A revision of the Schoenheimer-Sperry method for cholesterol determination. J Biol Chem. 1950 Nov;187(1):97–106. [PubMed] [Google Scholar]
- Torregrossa R. E., Makula R. A., Finnerty W. R. Characterization of lysocardiolipin from Acinetobacter sp. HO1-N. J Bacteriol. 1977 Aug;131(2):486–492. doi: 10.1128/jb.131.2.486-492.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. D., Rouser G. Precise quantitative determination of human blood lipids by thin-layer and triethylaminoethylcellulose column chromatography. I. Erythrocyte lipids. Anal Biochem. 1970 Dec;38(2):423–436. doi: 10.1016/0003-2697(70)90467-7. [DOI] [PubMed] [Google Scholar]
- Wadström T., Ljungh A., Wretlind B. Enterotoxin, haemolysin and cytotoxic protein in Aeromonas hydrophila from human infections. Acta Pathol Microbiol Scand B. 1976 Apr;84(2):112–114. doi: 10.1111/j.1699-0463.1976.tb01911.x. [DOI] [PubMed] [Google Scholar]
- Wretlind B., Hedén L., Wadström T. Formation of extracellular haemolysin by Aeromonas hydrophila in relation to protease and staphylolytic enzyme. J Gen Microbiol. 1973 Sep;78(1):57–65. doi: 10.1099/00221287-78-1-57. [DOI] [PubMed] [Google Scholar]
- Wretlind B., Möllby R., Wadström T. Separation of two hemolysins from Aeromonas hydrophila by isoelectric focusing. Infect Immun. 1971 Oct;4(4):503–505. doi: 10.1128/iai.4.4.503-505.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]




