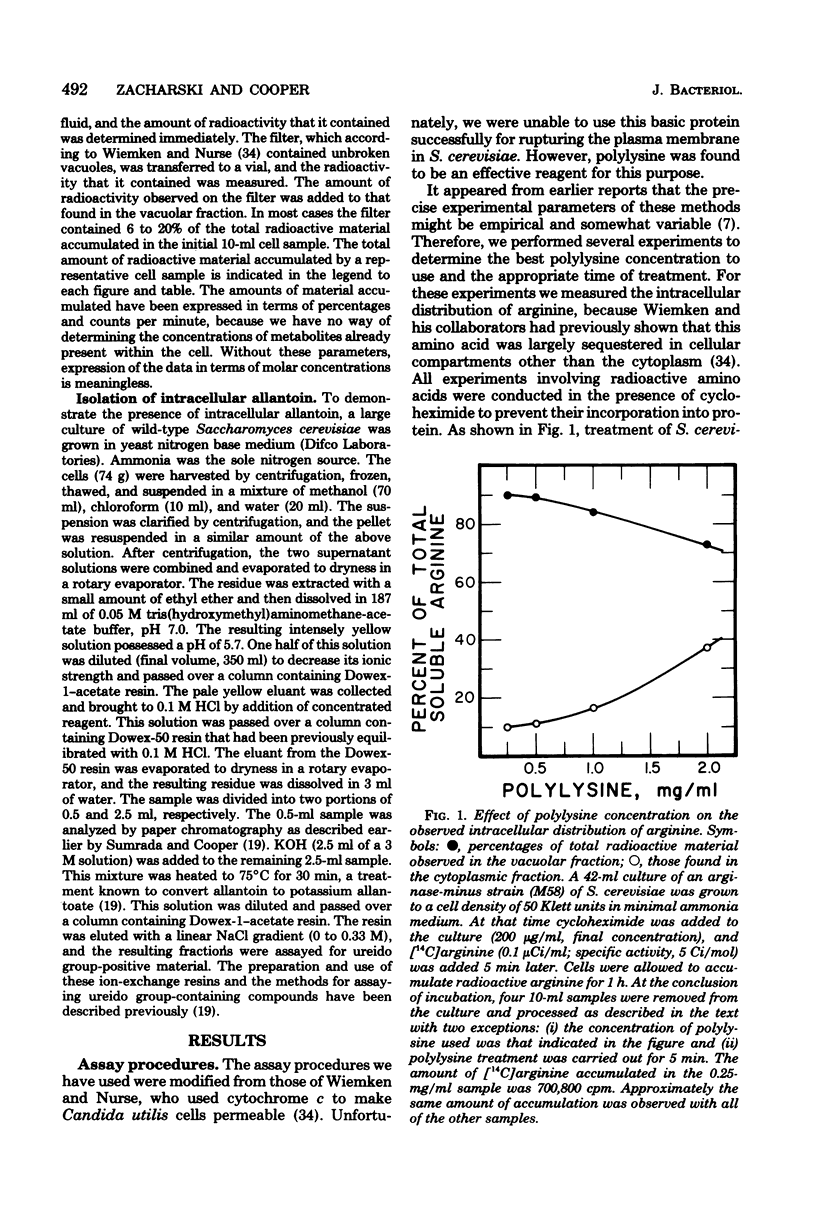
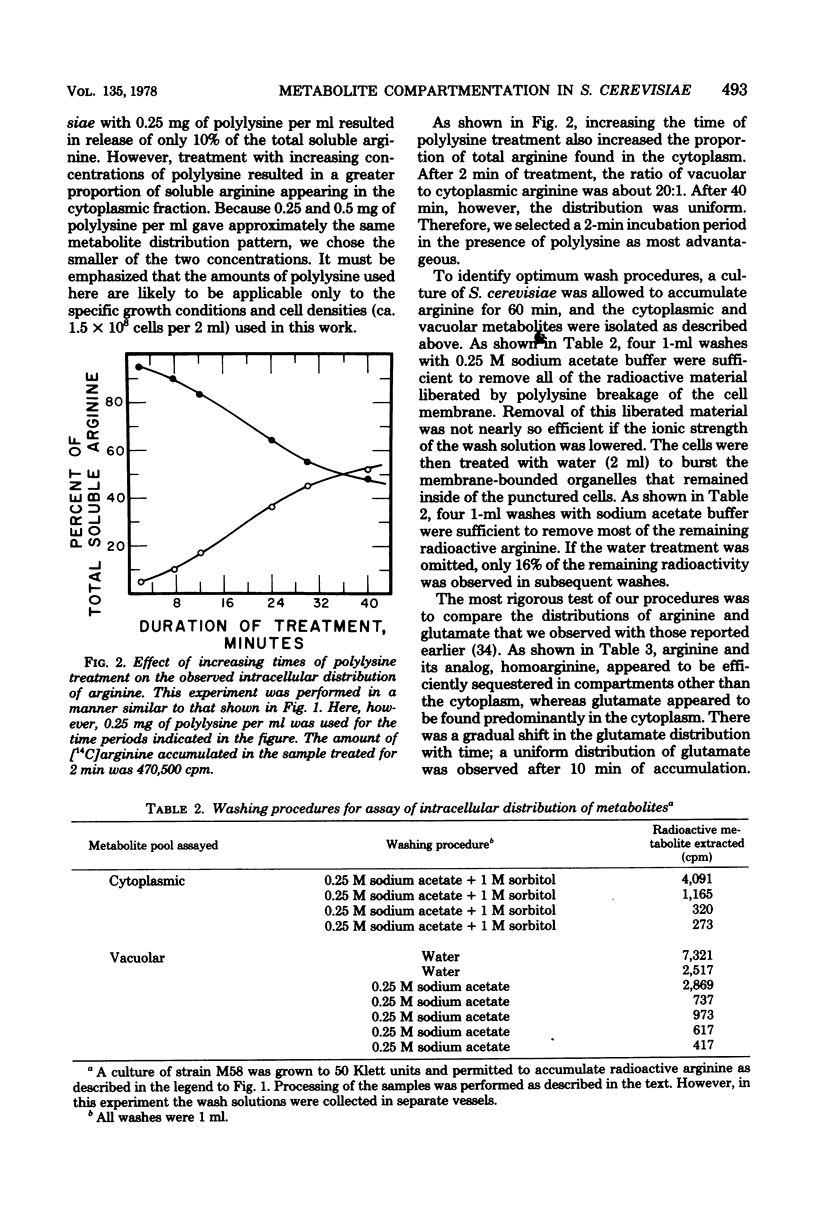
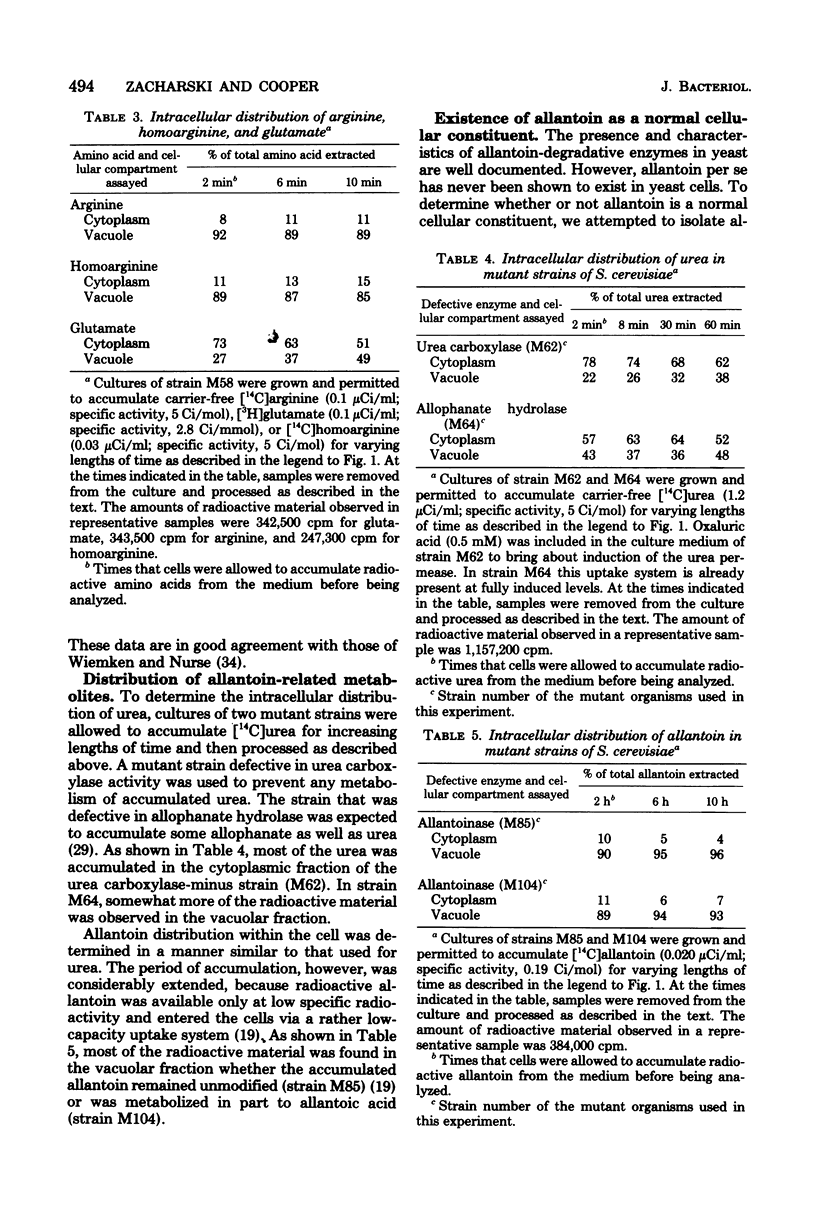
Abstract
Uninduced cultures of Saccharomyces cerevisiae exhibit high basal levels of allantoinase, allantoicase, and ureidoglycolate hydrolase, the enzymes responsible for degrading allantoin to urea. As a result, these activities increase only 4- to 8-fold upon induction, whereas the urea-degrading enzymes, urea carboxylase and allophanate hydrolase, have very low basal levels and routinely increase 30-fold on induction. Differences in the inducibility of these five enzymes were somewhat surprising because they are all part of the same pathway and have the same inducer, allophanate. Our current studies reconcile these observations. S. cerevisiae normally contained up to 1 mM allantoin sequestered in a cellular organelle, most likely the vacuole. Separation of the large amounts of allantoin and the enzymes that degrade it provide the cell with an efficient nitrogen reserve. On starvation, sequestered allantoin likely becomes accessible to these degradative enzymes. Because they are already present at high levels, the fact that their inducer is considerably removed from the input allantoin is of little consequence. This suggests that at times metabolite compartmentation may play an equal role with enzyme induction in the regulation of allantoin metabolism. Metabolism of arginine, another sequestered metabolite, must be controlled both by induction of arginase and compartmentation because arginine serves both as a reserve nitrogen source and a precursor of protein synthesis. The latter function precludes the existence of high basal levels of arginase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boller T., Dürr M., Wiemken A. Characterization of a specific transport system for arginine in isolated yeast vacuoles. Eur J Biochem. 1975 May;54(1):81–91. doi: 10.1111/j.1432-1033.1975.tb04116.x. [DOI] [PubMed] [Google Scholar]
- Bossinger J., Cooper T. G. Sequence of molecular events involved in induction of allophanate hydrolase. J Bacteriol. 1976 Apr;126(1):198–204. doi: 10.1128/jb.126.1.198-204.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybis J., Davis R. H. Organization and control in the arginine biosynthetic pathway of Neurospora. J Bacteriol. 1975 Jul;123(1):196–202. doi: 10.1128/jb.123.1.196-202.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. H. Utilization of exogenous and endogenous ornithine by Neurospora crassa. J Bacteriol. 1968 Aug;96(2):389–395. doi: 10.1128/jb.96.2.389-395.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürr M., Boller T., Wiemken A. Polybase induced lysis of yeast spheroplasts. A new gentle method for preparation of vacuoles. Arch Microbiol. 1975 Nov 7;105(3):319–327. doi: 10.1007/BF00447152. [DOI] [PubMed] [Google Scholar]
- Karlin J. N., Bowman B. J., Davis R. H. Compartmental behavior of ornithine in Neurospora crassa. J Biol Chem. 1976 Jul 10;251(13):3948–3955. [PubMed] [Google Scholar]
- Lawther R. P., Riemer E., Chojnacki B., Cooper T. G. Clustering of the genes for allantoin degradation in Saccharomyces cerevisiae. J Bacteriol. 1974 Aug;119(2):461–468. doi: 10.1128/jb.119.2.461-468.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matile P., Wiemken A. The vacuole as the lysosome of the yeast cell. Arch Mikrobiol. 1967 Feb 20;56(2):148–155. doi: 10.1007/BF00408765. [DOI] [PubMed] [Google Scholar]
- ROUSH A. H. Crystallization of purines in the vacuole of Candida utilis. Nature. 1961 Apr 29;190:449–449. doi: 10.1038/190449a0. [DOI] [PubMed] [Google Scholar]
- SCHLENK F., DAINKO J. L. ACTION OF RIBONUCLEASE PREPARATIONS ON VIABLE YEAST CELLS AND SPHEROPLASTS. J Bacteriol. 1965 Feb;89:428–436. doi: 10.1128/jb.89.2.428-436.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVIHLA G., DAINKO J. L., SCHLENK F. ULTRAVIOLET MICROSCOPY OF THE VACUOLE OF SACCHAROMYCES CEREVISIAE DURING SPORULATION. J Bacteriol. 1964 Aug;88:449–456. doi: 10.1128/jb.88.2.449-456.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVIHLA G., DAINKO J. L., SCHLENK F. Ultraviolet microscopy of purine compounds in the yeast vacuole. J Bacteriol. 1963 Feb;85:399–409. doi: 10.1128/jb.85.2.399-409.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVIHLA G., SCHLENK F. S-adenosylmethionine in the vacuole of Candida utilis. J Bacteriol. 1960 Jun;79:841–848. doi: 10.1128/jb.79.6.841-848.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlenk F., Dainko J. L., Svihla G. The accumulation and intracellular distribution of biological sulfoninum compounds in yeast. Arch Biochem Biophys. 1970 Sep;140(1):228–236. doi: 10.1016/0003-9861(70)90027-5. [DOI] [PubMed] [Google Scholar]
- Schlenk F., Zydek-Cwick C. R. Enzymatic activity of yeast cell ghosts produced by protein action on the membranes. Arch Biochem Biophys. 1970 May;138(1):220–225. doi: 10.1016/0003-9861(70)90301-2. [DOI] [PubMed] [Google Scholar]
- Schwencke J., De Robichon-Szulmajster H. The transport of S-adenosyl-L-methionine in isolated yeast vacuoles and spheroplasts. Eur J Biochem. 1976 May 17;65(1):49–60. doi: 10.1111/j.1432-1033.1976.tb10388.x. [DOI] [PubMed] [Google Scholar]
- Siegel S. M., Daly O. Regulation of betacyanin efflux from beet root by poly-L-lysine, ca-ion and other substances. Plant Physiol. 1966 Nov;41(9):1429–1434. doi: 10.1104/pp.41.9.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbing N. Growth and changes in pool and macromolecular components of Schizosaccharomyces pombe during the cell cycle. J Cell Sci. 1971 Nov;9(3):701–717. doi: 10.1242/jcs.9.3.701. [DOI] [PubMed] [Google Scholar]
- Subramanian K. N., Weiss R. L., Davis R. H. Use of external, biosynthetic, and organellar arginine by Neurospora. J Bacteriol. 1973 Jul;115(1):284–290. doi: 10.1128/jb.115.1.284-290.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumrada R., Zacharski C. A., Turoscy V., Cooper T. G. Induction and inhibition of the allantoin permease in Saccharomyces cerevisiae. J Bacteriol. 1978 Aug;135(2):498–510. doi: 10.1128/jb.135.2.498-510.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svihla G., Dainko J. L., Schlenk F. Ultraviolet micrography of penetration of exogenous cytochrome c into the yeast cell. J Bacteriol. 1969 Oct;100(1):498–504. doi: 10.1128/jb.100.1.498-504.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. L. Compartmentation and control of arginine metabolism in Neurospora. J Bacteriol. 1976 Jun;126(3):1173–1179. doi: 10.1128/jb.126.3.1173-1179.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. L., Davis R. H. Control of arginine utilization in Neurospora. J Bacteriol. 1977 Feb;129(2):866–873. doi: 10.1128/jb.129.2.866-873.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. L. Intracellular localization of ornithine and arginine pools in Neurospora. J Biol Chem. 1973 Aug 10;248(15):5409–5413. [PubMed] [Google Scholar]
- Whitney P. A., Cooper T. G., Magasanik B. The induction of urea carboxylase and allophanate hydrolase in Saccharomyces cerevisiae. J Biol Chem. 1973 Sep 10;248(17):6203–6209. [PubMed] [Google Scholar]
- Whitney P. A., Cooper T. G. Requirement for HCO3- by ATP: urea amido-lyase in yeast. Biochem Biophys Res Commun. 1970 Aug 24;40(4):814–819. doi: 10.1016/0006-291x(70)90975-7. [DOI] [PubMed] [Google Scholar]
- Whitney P. A., Cooper T. G. Urea carboxylase and allophanate hydrolase. Two components of adenosine triphosphate:urea amido-lyase in Saccharomyces cerevisiae. J Biol Chem. 1972 Mar 10;247(5):1349–1353. [PubMed] [Google Scholar]
- Wiemken A., Dürr M. Characterization of amino acid pools in the vacuolar compartment of Saccharomyces cerevisiae. Arch Microbiol. 1974;101(1):45–57. doi: 10.1007/BF00455924. [DOI] [PubMed] [Google Scholar]
- Wiemken A., Matile P., Moor H. Vacuolar dynamics in synchronously budding yeast. Arch Mikrobiol. 1970;70(2):89–103. doi: 10.1007/BF00412200. [DOI] [PubMed] [Google Scholar]
- Yphantis D. A., Dainko J. L., Schlenk F. Effect of some proteins on the yeast cell membrane. J Bacteriol. 1967 Nov;94(5):1509–1515. doi: 10.1128/jb.94.5.1509-1515.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


