Abstract
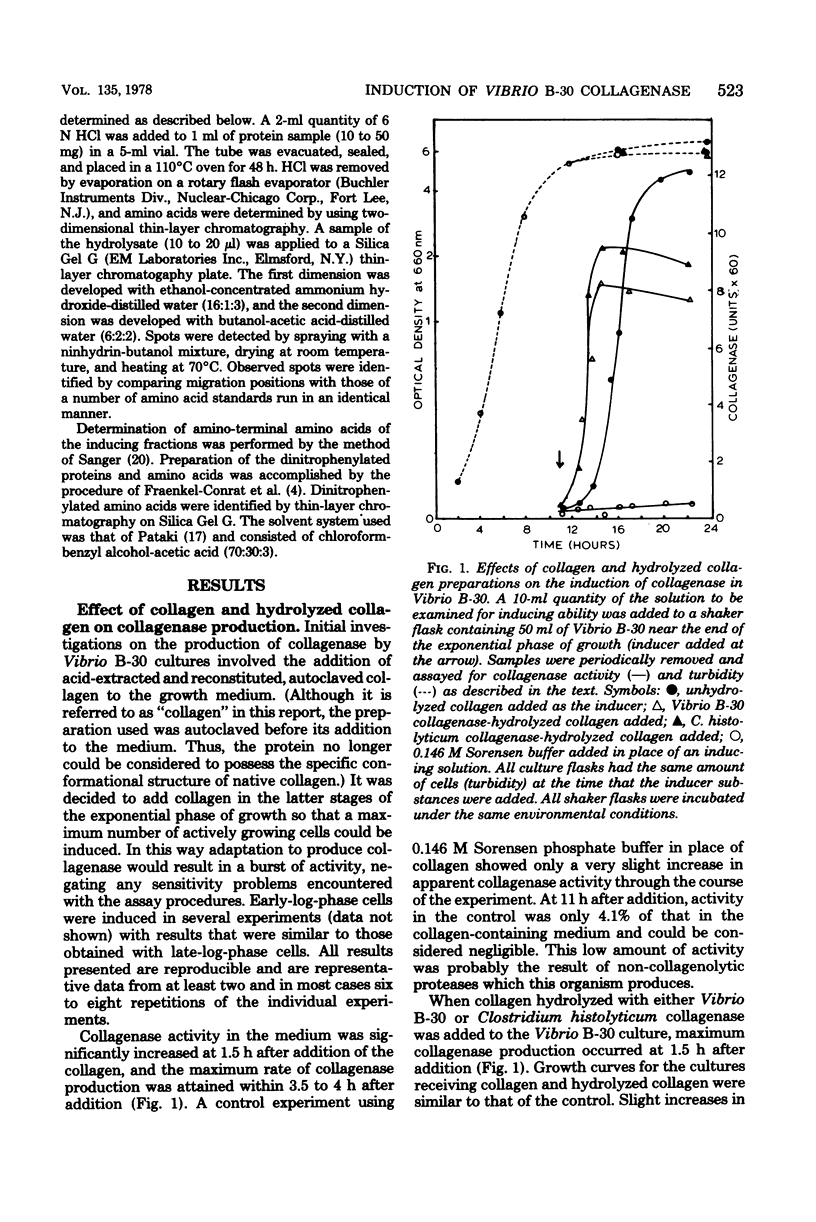
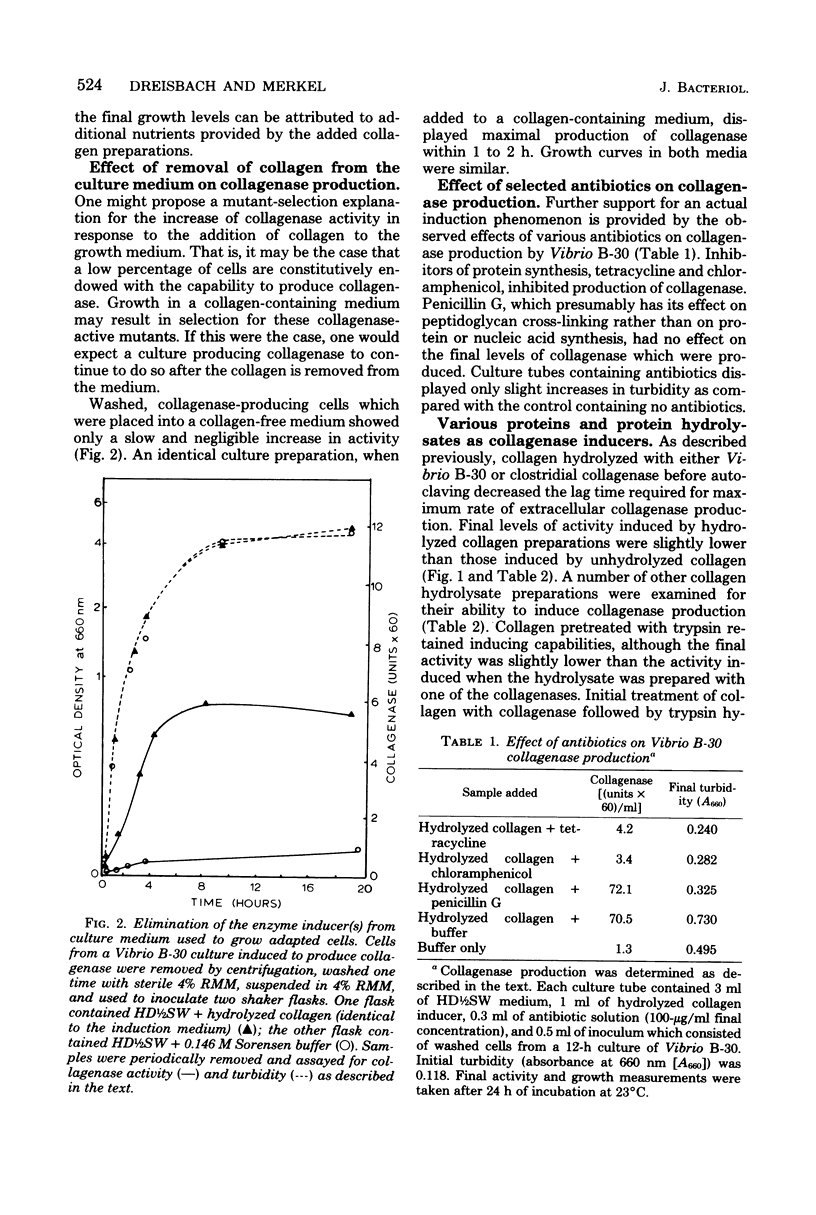
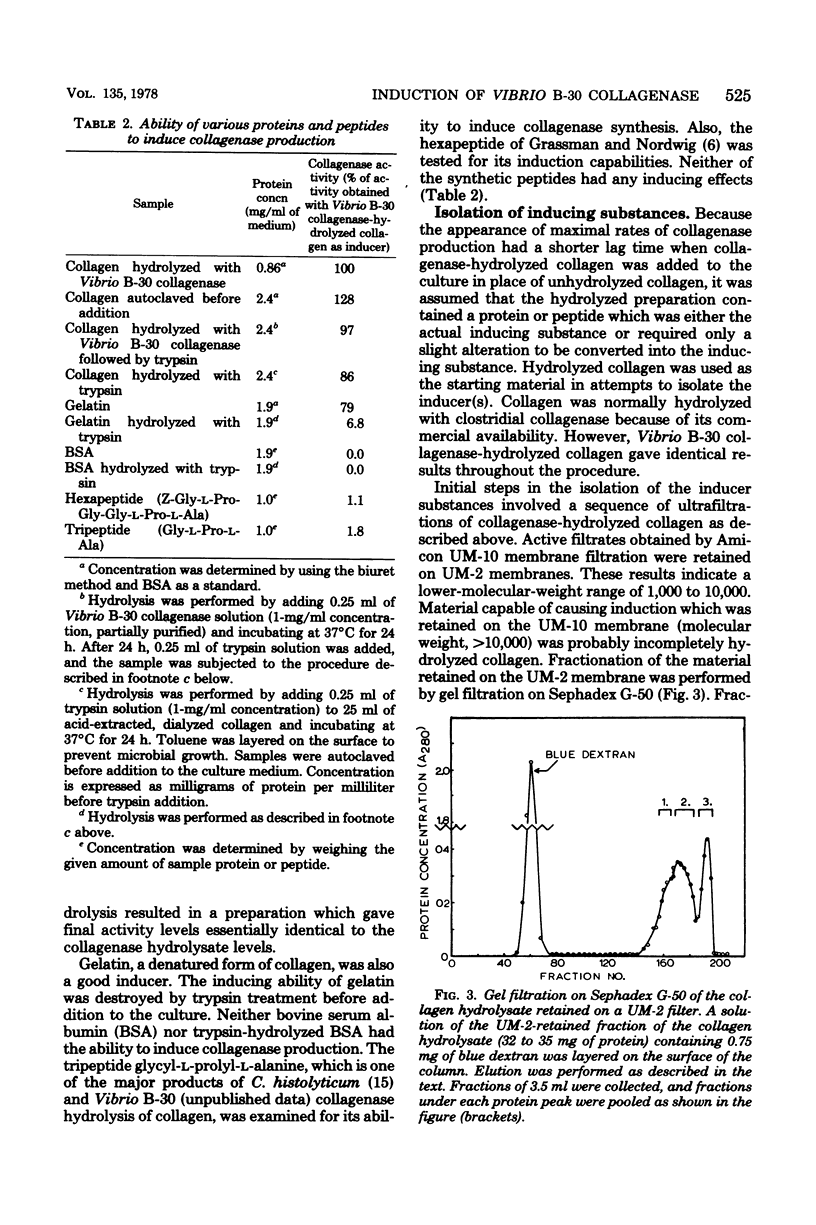
The inducible nature of an extracellular collagenase produced by a marine Vibrio (Vibrio B-30, ATCC 21250) was demonstrated by observing the increase in extracellular collagenase activity after the addition of collagen to cell cultures in the latter part of the exponential growth phase. When collagenase-hydrolyzed collagen was added, the lag time required before collagenase production was detected decreased significantly compared with cultures receiving collagen. Cells preinduced to synthesize collagenase did not produce the enzyme when collagen was removed from the culture medium. Incorporation of penicillin G had no effect on final collagenase activity levels in suspensions of Vibrio B-30 in complete medium supplemented with collagen. However, chloramphenicol and tetracycline inhibited collagenase production, indicating that de novo protein synthesis was necessary for the appearance of activity. Attempts to isolate the inducing substance(s) involved filtering hydrolyzed collagen through a series of ultrafiltration membranes. The lowest-molecular-weight fraction of collagen hydrolysate with inducing ability was between 1,000 and 10,000. Gel filtration of this fraction on Sephadex G-50 resulted in the appearance of three protein peaks, two of which were capable of inducing collagenase production. Results from amino acid composition and N-terminal amino acid analysis suggest that the inducing substance originates from the polar helical portion of the collagen molecule.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COHN M. Contributions of studies on the beta-galactosidase of Escherichia coli to our understanding of enzyme synthesis. Bacteriol Rev. 1957 Sep;21(3):140–168. doi: 10.1128/br.21.3.140-168.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daatselaar M. C., Harder W. Some aspects of the regulation of the production of extracellular proteolytic enzymes by a marine bacterium. Arch Microbiol. 1974;101(1):21–34. doi: 10.1007/BF00455922. [DOI] [PubMed] [Google Scholar]
- Drucker H. Regulation of exocellular proteases in Neurospora crassa: induction and repression of enzyme synthesis. J Bacteriol. 1972 Jun;110(3):1041–1049. doi: 10.1128/jb.110.3.1041-1049.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H., HARRIS J. I., LEVY A. L. Recent developments in techniques for terminal and sequence studies in peptides and proteins. Methods Biochem Anal. 1955;2:359–425. doi: 10.1002/9780470110188.ch12. [DOI] [PubMed] [Google Scholar]
- GRASSMANN W., NORDWIG A. [Quantitative colorimetric test for collagenase]. Hoppe Seylers Z Physiol Chem. 1960 Dec 31;322:267–272. doi: 10.1515/bchm2.1960.322.1.267. [DOI] [PubMed] [Google Scholar]
- GROSS J., KIRK D. The heat precipitation of collagen from neutral salt solutions: some rate-regulating factors. J Biol Chem. 1958 Aug;233(2):355–360. [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- Kühn K. The structure of collagen. Essays Biochem. 1969;5:59–87. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McDonald I. J., Chambers A. K. Regulation of proteinase formation in a species of Micrococcus. Can J Microbiol. 1966 Dec;12(6):1175–1185. doi: 10.1139/m66-159. [DOI] [PubMed] [Google Scholar]
- Merkel J. R., Dreisbach J. H., Ziegler H. B. Collagenolytic activity of some marine bacteria. Appl Microbiol. 1975 Feb;29(2):145–151. doi: 10.1128/am.29.2.145-151.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGAI Y., NODA H. The specificity of collagenase. Biochim Biophys Acta. 1959 Jul;34:298–299. doi: 10.1016/0006-3002(59)90280-x. [DOI] [PubMed] [Google Scholar]
- Nordwig A. Collagenolytic enzymes. Adv Enzymol Relat Areas Mol Biol. 1971;34:155–205. doi: 10.1002/9780470122792.ch4. [DOI] [PubMed] [Google Scholar]
- ROSEN H. A modified ninhydrin colorimetric analysis for amino acids. Arch Biochem Biophys. 1957 Mar;67(1):10–15. doi: 10.1016/0003-9861(57)90241-2. [DOI] [PubMed] [Google Scholar]
- SANGER F. The terminal peptides of insulin. Biochem J. 1949;45(5):563–574. doi: 10.1042/bj0450563. [DOI] [PMC free article] [PubMed] [Google Scholar]


