Abstract
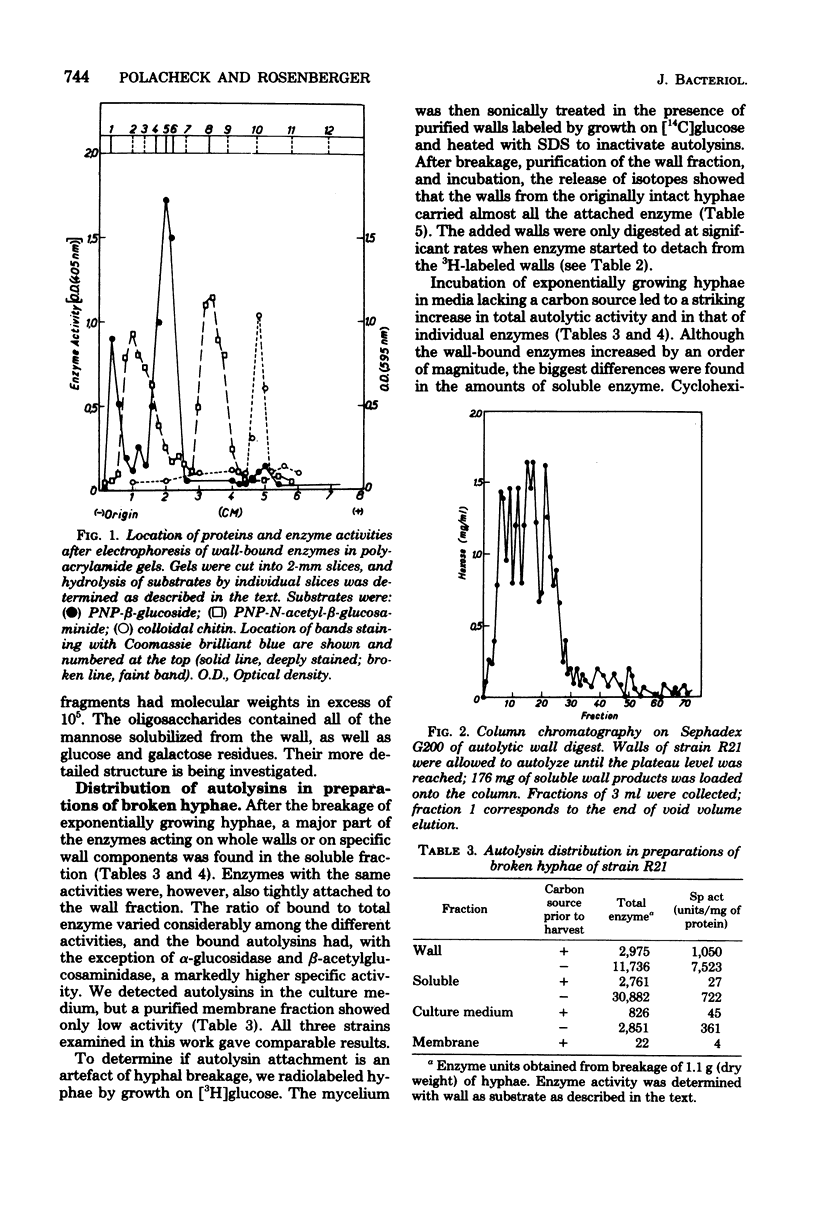
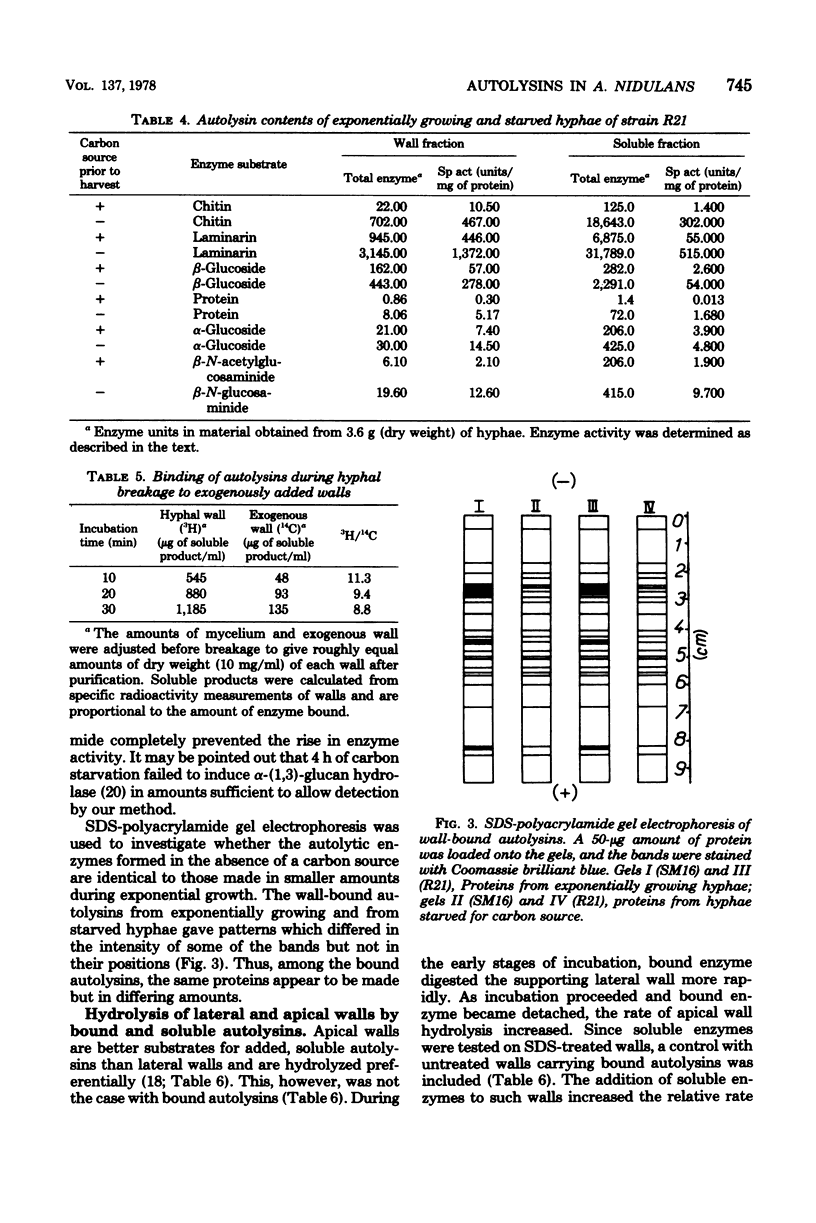
Preparations of broken Aspergillus nidulans hyphae contained both free and wall-bound autolysins. The bound enzymes were not solubilized by 8 M LiCl or neutral or anionic detergents; they were readily detached from walls by a cationic detergent or by autodigestion. Once detached, the enzymes did not reassociate with wall to give salt-resistant complexes. Six enzymes hydrolyzing wall polymers were bound to the envelope, and the same activities were also detected among soluble proteins in the cytoplasmic fraction. It is suggested that cytoplasmic vesicles, containing autolysins, are inserted into or trapped by newly formed wall in the growing hypha; these constitute the wall-bound autolysin fraction. Starvation for a carbon source derepressed the synthesis of five out of the six autolysins, and the amounts of both soluble and wall-bound activities increased by one to two orders of magnitude.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERGER L. R., REYNOLDS D. M. The chitinase system of a strain of Streptomyces griseus. Biochim Biophys Acta. 1958 Sep;29(3):522–534. doi: 10.1016/0006-3002(58)90008-8. [DOI] [PubMed] [Google Scholar]
- Cabib E., Farkas V. The control of morphogenesis: an enzymatic mechanism for the initiation of septum formation in yeast. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2052–2056. doi: 10.1073/pnas.68.9.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib E., Ulane R., Bowers B. Yeast chitin synthetase. Separation of the zymogen from its activating factor and recovery of the latter in the vacuole fraction. J Biol Chem. 1973 Feb 25;248(4):1451–1458. [PubMed] [Google Scholar]
- Chrambach A., Reisfeld R. A., Wyckoff M., Zaccari J. A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Jul;20(1):150–154. doi: 10.1016/0003-2697(67)90272-2. [DOI] [PubMed] [Google Scholar]
- Cohen B. L. Regulation of intracellular and extracellular neutral and alkaline proteases in Aspergillus nidulans. J Gen Microbiol. 1973 Dec;79(2):311–320. doi: 10.1099/00221287-79-2-311. [DOI] [PubMed] [Google Scholar]
- Dygert S., Li L. H., Florida D., Thoma J. A. Determination of reducing sugar with improved precision. Anal Biochem. 1965 Dec;13(3):367–374. doi: 10.1016/0003-2697(65)90327-1. [DOI] [PubMed] [Google Scholar]
- Fleet G. H., Phaff H. J. Glucanases in Schizosaccharomyces. Isolation and properties of the cell wall-associated beta(1 leads to 3)-glucanases. J Biol Chem. 1974 Mar 25;249(6):1717–1728. [PubMed] [Google Scholar]
- Fleming H., Haselkorn R. The program of protein synthesis during heterocyst differentiation in nitrogen-fixing blue-green algae. Cell. 1974 Oct;3(2):169–170. doi: 10.1016/0092-8674(74)90121-4. [DOI] [PubMed] [Google Scholar]
- Grove S. N., Bracker C. E. Protoplasmic organization of hyphal tips among fungi: vesicles and Spitzenkörper. J Bacteriol. 1970 Nov;104(2):989–1009. doi: 10.1128/jb.104.2.989-1009.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevan P. R., Mahadkar U. R. Role of enzymes in growth and morphology of Neurospora crassa: cell-wall-bound enzymes and their possible role in branching. J Bacteriol. 1970 Mar;101(3):941–947. doi: 10.1128/jb.101.3.941-947.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matile P., Cortat M., Wiemken A., Frey-Wyssling A. Isolation of glucanase-containing particles from budding Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1971 Mar;68(3):636–640. doi: 10.1073/pnas.68.3.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page W. J., Stock J. J. Isolation and characterization of Microsporum gypseum lysosomes: role of lysosomes in macroconidia germination. J Bacteriol. 1972 Apr;110(1):354–362. doi: 10.1128/jb.110.1.354-362.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polacheck I., Rosenberger R. F. Aspergillus nidulans mutant lacking alpha-(1,3)-glucan, melanin, and cleistothecia. J Bacteriol. 1977 Nov;132(2):650–656. doi: 10.1128/jb.132.2.650-656.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polacheck Y., Rosengerger R. F. Autolytic enzymes in hyphae of Aspergillus nidulans: their action on old and newly formed walls. J Bacteriol. 1975 Jan;121(1):332–337. doi: 10.1128/jb.121.1.332-337.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonneveld B. J. A new type of enzyme, and exo-splitting -1,3 glucanase from non-induced cultures of Aspergillus nidulans. Biochim Biophys Acta. 1972 Feb 28;258(2):541–547. doi: 10.1016/0005-2744(72)90245-8. [DOI] [PubMed] [Google Scholar]
- Zonneveld B. J. Morphogenesis in Aspergillus nidulans. The significance of a alpha-1, 3-glucan of the cell wall and alpha-1, 3-glucanase for cleistothecium development. Biochim Biophys Acta. 1972 Jun 26;273(1):174–187. doi: 10.1016/0304-4165(72)90205-x. [DOI] [PubMed] [Google Scholar]


