Abstract
Recombinant adeno-associated virus (rAAV) is a promising vector for gene therapy. Recent isolations of novel AAV serotypes have led to significant advances by broadening the tropism and increasing the efficiency of gene transfer to the desired target cell. However, a major concern that remains is the strong preexisting immune responses to several vectors. In this paper, we describe the isolation and characterization of AAV12, an AAV serotype with unique biological and immunological properties. In contrast to those of all other reported AAVs, AAV12 cell attachment and transduction do not require cell surface sialic acids or heparan sulfate proteoglycans. Furthermore, rAAV12 is resistant to neutralization by circulating antibodies from human serum. The feasibility of rAAV12 as a vector was demonstrated in a mouse model in which muscle and salivary glands were transduced. These characteristics make rAAV12 an interesting candidate for gene transfer applications.
Adeno-associated virus (AAV) is a nonpathogenic human parvovirus originally described in 1965 by Atchison et al. as a contaminant in a preparation of simian adenovirus 15 (2). AAV's close relationship with adenovirus has been a hallmark of this genus. AAVs require the presence of a helper virus for efficient replication and virus production. Most studies thus far have analyzed the helper functions of adenovirus and herpesvirus. However, cytomegalovirus (17), Epstein-Barr virus, varicella virus (10), papillomavirus (18, 29), pseudorabies virus (19), and vaccinia virus (20) are described as potential helper viruses for AAV replication.
Because of this close relationship between AAV and its helper viruses, a number of different AAV isolates have been found in preparations of adenoviruses. AAVs have been detected in primate and nonprimate adenovirus stocks, including isolates from bovine, avian, ovine, and equine origins (1).
We recently reported the identification of several new isolates from adenoviral stocks held by the American Type Culture Collection (ATCC) with high sequence similarity to AAV1 and AAV6 (22). However, despite their close sequence similarity, they also display unique biologic activities. Characterization of several of these isolates identified differences in their responses to preexisting antibodies and entry pathways as well as kinetics of uncoating and transgene expression. Due to these differences in biologic activity, AAV vectors are considered a very diverse platform for gene transfer. For example, AAV1 vectors very efficiently transduce skeletal muscle and other tissues whereas AAV4 demonstrates very specific and high-level transduction of ependymal cells in the central nervous system (7, 8).
In part, this unique cell tropism can be attributed to the differences in cell attachment receptors used for AAV binding and entry. AAVs utilize a diverse array of cell surface carbohydrates for attachment and infection. AAV2 has been shown to bind heparan sulfate proteoglycans (HSPGs) on the cell surface (26). Competition experiments have demonstrated that soluble heparin can block virus binding and transduction. Furthermore, differentiated airway lung epithelial cells, which express very little HSPG on their apical surfaces, are poorly transduced. Not all AAVs interact with HSPGs. AAV4 and AAV5 both use different forms of sialic acid for cell attachment. While both AAV4 and AAV5 require α2,3-linked sialic acid for cell attachment and transduction, they differ in their linkage specificity. AAV4 preferentially attaches to α2,3 sialic acid present on the O-linked carbohydrate core, while AAV5 attached to the N-linked type (12, 28). AAV1 and AAV6 also have been reported to use either α2,3 or α2,6 N-linked sialic acid for binding (30). Bovine AAV also requires sialic acid for attachment but for cell entry requires a different form of sialic acid that is found in gangliosides, ceramide-based glycolipids containing one or more sialic acid groups (21).
In this report, we characterize a new AAV serotype, AAV12, which was isolated from a simian adenovirus 18 stock. The sequence of the rep gene places it in the AAV2 complementation group, but the capsid is only 60% identical to that of AAV2. Our characterization of this isolate revealed the unique biological and antigenic properties of recombinant AAV12 (rAAV12). Unlike those of all other described AAVs, rAAV12 cell attachment and transduction are independent of both HSPG and sialic acid, resulting in a unique transduction profile in vitro. The feasibility of rAAVs as vectors for in vivo gene transfer was demonstrated in a mouse model. These features of rAAV12, together with a high resistance to neutralization by human serum, render rAAV12 an interesting candidate for gene transfer applications.
MATERIALS AND METHODS
Cell culture and virus.
African green monkey kidney COS cells obtained from the American Type Culture Collection (ATCC) (Manassas, VA) were cultured in RPMI 1640 medium (Biosource, Camarillo, CA) supplemented with 5% fetal bovine serum (HyClone, Logan, UT), 2 mM l-glutamine, 100 U of penicillin/ml, and 0.1 mg of streptomycin/ml (Invitrogen, Carlsbad, CA). Cells were maintained at 37°C under a 5% CO2 humidified atmosphere. The simian virus 18 isolate VR-943 was obtained from the ATCC as a crude lysate of virus-infected cells.
Subcloning of the AAV12 rep and cap genes.
The complete coding regions of AAV12 rep and cap were PCR amplified and subcloned. DNA was isolated from lysates of simian virus 18-infected cells with a QIAprep Spin mini prep kit (Qiagen, Valencia, CA).The rep open reading frame (ORF) was PCR amplified from this DNA with the primers AAV225(+) (GCGACAKTTTGCGACACCAYGTGG) and UNI-NC (CCANNNGGAATCGCAATGCCAAT). AAV12 cap was amplified with the primers UNI-C (5′-ATGNTNATNTGGTGGGAGGAGGG-3′) and AAV1-4 polyA4400(−) (5′-CGAATNAAMCGGTTTATTGATTAAC-3′). The PCR fragments were subcloned using a TOPO TA cloning kit (Invitrogen), resulting in the plasmids pAAV12Rep and pAAV12Cap. Three clones that were capable of generating recombinant virus were sequenced with an ABI Prism 3100 genetic analyzer (Applied Biosystems, Foster City, CA) and FS dye terminator chemistry (Applied Biosystems).
Sequence analysis.
The sequences of AAV12 rep and cap were compared to sequences in GenBank by using BLAST. DNA alignments were performed using the ClustalW multiple sequence alignment tool of the Biology Workbench Web-based software at http://seqtool.sdsc.edu/ (SDSC) and MacVector 7 (Accelrys, Burlington, MA).
Generation of recombinant virus and transduction of NCI60 cells.
AAV12 vectors expressing a nuclear-localized green fluorescent protein (GFP) (rAAV12-GFP) were produced as described earlier (23). Briefly, 293T cells were cotransfected with pAAV2-NLS-GFP, pAAV12Rep, and pAAV12Cap and the adenovirus helper plasmid 449B (25). Recombinant particles were purified by CsCl gradient centrifugation. Production of rAAV2-GFP, rAAV4-GFP, and rAAV5-GFP has been described earlier (13). The DNase-resistant genome copy numbers of the vector stocks were determined by quantitative real-time PCR using the TaqMan system (Applied Biosystems) with probes specific to the cytomegalovirus promoter. AAV12-Epo expressing human erythropoietin (hEPO) was generated accordingly by packaging pAAVhEPO (27) with pAAV12Rep and pAAV12Cap. Production of AAV2-Epo was previously described (27). NCI60 cancer cells were transduced by plating 1 × 104 cells/well in a flat-bottom 96-well plate. Twenty-four hours after seeding, cells were transduced with 2 × 107 particles of rAAV4-GFP or rAAV12-GFP. GFP-positive cells were counted by flow cytometry at 24 h posttransduction.
Digestion of cell surface sialic acid.
Exponentially growing COS cells were plated at a density of 5 × 103/well in a flat-bottom 96-well plate. Twenty-four hours after seeding, cells were incubated for 30 min with 0.1 or 1 mU of the broad-spectrum neuraminidase from Vibrio cholerae (Calbiochem, La Jolla, CA) to remove sialic acid. Cells were then washed with medium and transduced with 1 × 107 particles of rAAV2-GFP, rAAV4-GFP, rAAV5-GFP, and rAAV12-GFP. GFP expression, which serves as a surrogate marker for transduction, was detected 42 h later with a fluorescent cell counter (Guava PCA-96; Guava Technologies, Hayward, CA).
Heparin competition assay.
COS cells were plated at a density of 5 × 103/well in a flat-bottom 96-well plate 1 day prior to transduction. Particles (2 × 107) of rAAV2-GFP or rAAV12-GFP were preincubated for 1 h at room temperature in medium supplemented with heparin (Sigma, St. Louis, MO). This preincubation mixture was then added and left on the cells for 1 h at 37°C. Cells were then washed with medium and incubated for an additional day before GFP expression was detected with a fluorescent cell counter (Guava Technologies).
Protease treatment.
COS cells were cultured in a 15-cm-diameter culture dish until cells were 80% confluent. Cells were then washed twice with phosphate-buffered saline, scraped, resuspended in 10 ml phosphate-buffered saline, and incubated with 0.05% trypsin (Biosource) or mock medium (untreated control) for 15 min at 37°C. Cells were washed twice with medium and seeded at a density of 10,000 cells/well in a 96-well dish. After 1 h of culture at 37°C, cells were transduced with 2 × 107 particles of rAAV2-GFP or rAAV12-GFP. Transduction efficiency was determined 24 h later by GFP expression detection with a fluorescent cell counter (Guava Technologies).
Inhibition of glycolipid synthesis.
COS cells were plated at a density of 5 × 103/well in a flat-bottom 96-well plate. Eight hours after seeding, cells were incubated for 40 h with the glucosylceramide synthase inhibitor dl-threo-1-phenyl-2-palmitoylamino-3-morpholino-1-propanol (PPMP) (Sigma, St. Louis, MO). Cells were then washed with medium and transduced with 2 ×108 particles of rAAV2-GFP, rAAV12-GFP, or recombinant bovine AAV (rBAAV)-GFP for 1 h. GFP expression was analyzed 48 h after transduction by detection with a fluorescent cell counter (Guava Technologies).
Neutralization assay.
COS cells were seeded at a density of 5 × 103/well in a 96-well plate 1 day before inoculation with 2 × 107 rAAV2-GFP, rAAV6-GFP, or rAAV12-GFP particles that were preincubated with serial dilutions of pooled human immunoglobulin Gs (IgGs) (Gamunex [Immune Globulin Intravenous 10%]; Bayer, Leverkusen, Germany) in medium for 1 h at room temperature. Cells were exposed for 1 h at 37°C and then washed with medium. Twenty-four hours after transduction, cells were analyzed for GFP expression by flow cytometry (Guava Technologies).
Inhibition of endosomal acidification.
COS cells were seeded at a density of 5 × 103/well in a 96-well plate 1 day before inoculation. Cells were preincubated with either mock medium, 5 nM bafilomycin A1, or 10 mM or 20 mM NH4Cl for 30 min before being transduced for 1 h in inhibitor containing medium with 2 × 107 rAAV2-GFP and rAAV12-GFP particles. Twenty-four hours after transduction, cells were analyzed for GFP expression by flow cytometry (Guava Technologies).
Animal experiments.
Animal studies were approved by the NIDCR Animal Care and Use Committee and the NIH Biosafety Committee. All procedures were conducted in accordance with IASP standards. Male BALB/c mice were obtained from the Division of Cancer Treatment, NCI, Bethesda, MD. Mice were administered 109 particles (suspended in 50 μl of 0.9% NaCl) of either an AAV2 vector encoding hEPO (AAV2-hEPO) (n = 3) or AAV12-hEPO (n = 2) by retrograde ductal delivery to both submandibular glands (3, 5). Two additional groups (n = 3 and n = 2) received equal doses of 109 particles (suspended in 50 μl of 0.9% NaCl) in both tibialis anterior muscles (two injection sites per muscle). Another group (n = 3) of naïve mice (administered 50 μl of 0.9% NaCl in each submandibular gland) was included. Mild anesthesia was induced in all participating animals with 1 μl/g body weight of 60 mg/ml ketamine (Phoenix Scientific, St. Joseph, MO) and 8 mg/ml xylazine (Phoenix Scientific) given intramuscularly. Blood samples were obtained by orbital bleeding at different time points. Hematocrits were determined using microhematocrit capillary tubes (Fisher Scientific, Pittsburgh, PA). Secretion of hEPO in mouse serum was determined by an enzyme-linked immunosorbent assay using commercial assay kits (R&D Systems, MN). The lower limit of detection was 0.6 mU/ml. The assays were performed according to the manufacturer's instructions.
Nucleotide sequence accession number.
The sequence determined in this study has been deposited in GenBank under accession number DQ813647.
RESULTS
Identification of AAV contaminations in ATCC virus isolates.
We have previously analyzed the ATCC virus collection for AAV contamination (14, 22). To continue this, we detected a strong PCR signal with our AAV genome-specific probes by using PCR of DNA purified from a simian adenovirus 18 strain C676 stock, VR-943, which was isolated from a Vervet monkey (Cercopithecus aethiops). By use of multiple PCRs specific for different regions of the AAV genome, the entire rep and cap coding regions of the AAV contamination in VR-943, termed AAV12, were amplified and subcloned. Since the rep and cap regions encoding PCR fragments also contain viral promoter elements, these plasmids could be used as packaging constructs for the generation of recombinant virus. Initial experiments demonstrated that AAV12 rep could be used to replicate AAV2 inverted terminal repeat-containing plasmids (data not shown). Vectors based on AAV12 were produced by cotransfecting an AAV2 vector plasmid encoding a nuclear-localized GFP flanked by AAV2 inverted terminal repeats and plasmids carrying AAV12 rep and cap, together with an adenovirus helper plasmid. Recombinant viruses were then assayed for transduction activity by inoculating COS cells and assaying for GFP expression by flow cytometry. Three clones that were capable of generating recombinant virus were sequenced and further analyzed.
Phylogenetic analysis.
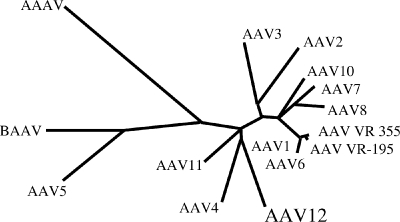
The evolutionary relationship among mammalian AAVs and AAV12 was analyzed by ClustalW alignments of genomic, Rep78, and VP1 sequences and plotted as a rooted phylogenetic tree (Fig. 1). The genome DNA sequence of AAV12 showed the highest homologies with those of AAV11 and AAV4, at 83% and 81%, respectively, whereas the lowest similarity was observed with that of AAV5 (63%). The Rep78 amino acid sequence of AAV12 demonstrated high homology to those of AAV4 and AAV11, with 89% to 88% identity, respectively, with the majority of the differences being located in the carboxy-terminal 100 amino acids. While these changes had little effect on the spliced Rep products (Rep68 and Rep40), they altered the activities of the unspliced proteins Rep78 and Rep52. For the capsid protein VP1, the highest homologies were observed with AAV11 and AAV4 (84% and 78%, respectively), whereas AAV5 VP1 displayed the lowest similarity, with 53% homology. Unlike for the Rep ORF, these mutations were scattered about the Cap ORF.
FIG. 1.
Evolutionary relationship among human and nonhuman primate AAVs and AAV12. The unrooted phylogenetic tree is based on merged ClustalW alignments of partial genome sequences and shows the relatedness of different AAVs; the lengths of the branches are proportional to the evolutionary distances between isolates.
Heparin competition.
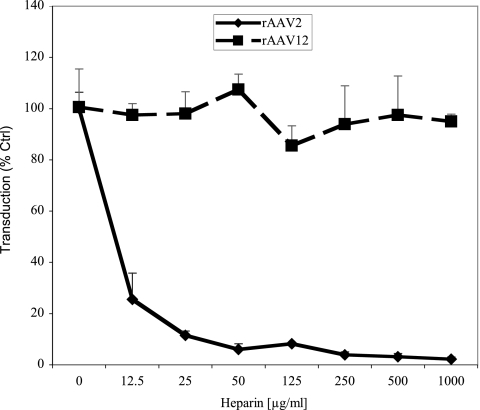
Heparan sulfate, a cell surface glycosaminoglycan expressed by virtually all cells, is an attachment receptor for AAV2 (26). AAV2 transduction can be inhibited by heparin, a heparan sulfate analog. Therefore, by heparin competition experiments, we analyzed whether AAV12 uses heparan sulfate as a receptor. COS cells were transduced with rAAV12 in the presence of increasing amounts of heparin, using rAAV2 as a control. While rAAV2 transduction was inhibited by 75% at a concentration of 12.5 μg/ml heparin, no inhibition of rAAV12 was observed even at an 80-fold-higher heparin concentration (1,000 μg/ml) (Fig. 2). Since heparin has no inhibitory potential toward AAV12 transduction, HSPGs do not appear to be involved in AAV12 transduction.
FIG. 2.
rAAV12 COS cell transduction is not inhibited by heparin. COS cells were transduced with a preincubation mixture consisting of rAAV2-GFP or rAAV12-GFP, and heparin was added at the indicated concentrations. Transduction was analyzed by flow cytometry at 24 h postinoculation. Values are the means from three experiments; error bars represent standard deviations.
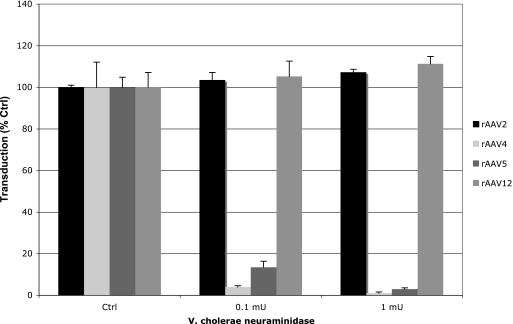
Effect of neuraminidase treatment on rAAV12 transduction.
Sialic acid, a monosaccharide based on N-acetylneuramic acid, is commonly found on the outermost ends of glycans and glycolipids and serves as a receptor for several viruses, including influenza virus, AAV4, AAV5, AAV6, and AAV(VR-355) (12, 22, 28, 30). To analyze whether AAV12 transduction is sialic acid dependent, cell surface sialic acid was removed from COS cells by treatment with neuraminidase prior to transduction. The transduction efficiencies in treated cells were compared to those in untreated controls (Fig. 3). Enzymatic digestion of COS cells with a broad-spectrum neuraminidase from Vibrio cholera inhibited rAAV4 and rAAV5 transduction in a dose-dependent manner and blocked gene transfer up to 99% and 97%, respectively. AAV12 transduction was unaffected by the enzymatic removal of cell surface sialic acids, suggesting that AAV12 does not utilize sialic acid for binding or entry.
FIG. 3.
rAAV12 transduction is independent of cell surface sialic acid. COS cells were pretreated with Vibrio cholera neuraminidase to remove exposed sialic acid groups before the cells were transduced with rAAV2-GFP, rAAV4-GFP, rAAV5-GFP, or rAAV12-GFP. Gene transfer was determined by flow cytometry. Values are means compared to levels for untreated control cells from three experiments; error bars represent standard deviations.
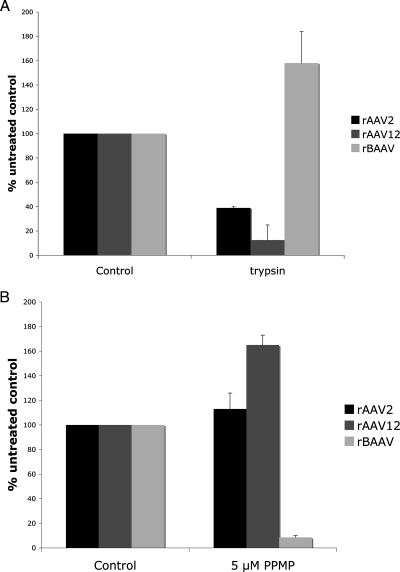
rAAV12 transduction is protease sensitive and does not require glycosphingolipids.
We recently demonstrated that rBAAV cell entry and transduction depend on gangliosides, glycosphingolipids with sialic acid groups. Furthermore, rBAAV entry is resistant to protease treatment of the cell (21). To analyze whether the rAAV12 receptor complex contains a protein component and whether glycolipids are involved in the transduction process, target cells were treated with either proteases or dl-threo-1-phenyl-2-palmitoylamino-3-morpholino-1-propanol (PPMP), a glucosylceramide synthase inhibitor that depletes glycosphingolipids from the cell membrane (16) prior to transduction (Fig. 4A or B, respectively). Proteolytic digestion of cell surface proteins inhibited rAAV12 transduction by 90%, suggesting that like AAV2, cell surface proteins are important in AAV12 entry. In contrast, treatment with PPMP did not inhibit AAV12 transduction, indicating that glycolipids were not involved in AAV12 transduction.
FIG. 4.
rAAV12 transduction is protease sensitive and does not require glycosphingolipids. COS cells were proteolytically digested with trypsin (A) or treated with the glycosphingolipid synthesis inhibitor PPMP (B) prior to transduction with rAAV2-GFP, rAAV12-GFP, or rBAAV-GFP. Gene transfer in these cells was compared to that in untreated cultures. Values are means from three experiments; error bars represent standard deviations.
Like AAV2, rAAV12 requires endosomal acidification for transduction.
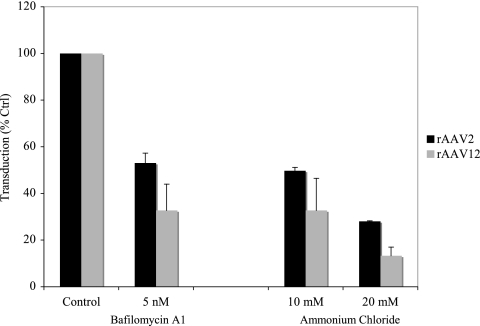
AAV2 cell entry is mediated by receptor-mediated endocytosis. Release from the endosomes requires endosomal acidification (4). To analyze whether AAV12 also depends on an acidification step during transduction, we analyzed the effects of drugs that can interfere with the endosomal pH. Bafilomycin A1, an inhibitor of the vacuolar H+-ATPase that is responsible for acidification of endosomal vesicles, inhibited AAV2 and rAAV12 transduction by 65% at a 5 nM concentration. NH4Cl, a lysosomotropic weak base, which raises the pHs within vesicles by functioning as a proton sink (15), also inhibited over 70% of rAAV2 and rAAV12 transduction activity (Fig. 5). Therefore, while AAV2 and AAV12 appear to bind distinct cell surface receptors, they both require endosomal acidification for transduction.
FIG. 5.
rAAV12 transduction requires endosomal acidification. COS cells were preincubated with 5 nM bafilomycin A1 or 10 mM or 20 mM ammonium chloride to inhibit endosomal acidification prior to transduction with rAAV2 or rAAV12. Transduction efficiencies in treated cells were compared to those in the untreated control. Values are means from three experiments; error bars represent standard deviations.
rAAV12 has a broad tropism in human cancer cell lines.
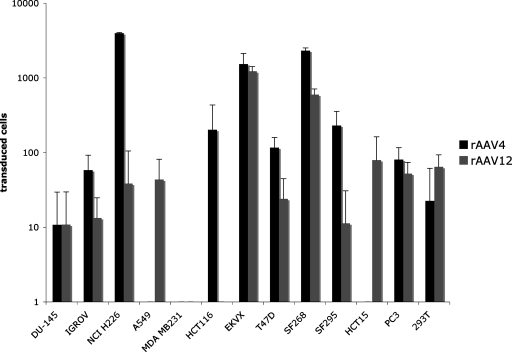
In contrast to all other studied AAVs, rAAV12 appears not to utilize either sialic acid or heparin as a cellular receptor or attachment factor. To analyze whether AAV12's unique cell interaction also results in a unique tropism, we compared the transduction efficiency of rAAV12 to that of rAAV4, an AAV serotype with the highest homology (78%) to the AAV12 capsid in 13 cell lines from a variety of tissues (Fig. 6). rAAV12 and rAAV4 demonstrated distinct but overlapping transduction activity. For example, AAV4 uniquely transduced HCT116 cells, while only rAAV12 gene transfer activity was observed with A549 and HCT15 cells.
FIG. 6.
rAAV12 has a broad tropism. The transduction efficiency of rAAV12 was compared to that of rAAV4 in 13 human cancer cell lines. Cells were transduced with either rAAV12-GFP or rAAV4-GFP. Transduction was analyzed by flow cytometry 28 h after virus inoculation. Values are means from three experiments; error bars represent standard deviations.
Immunological characterization of rAAV12.
The clinical utility of AAV-based vectors may be limited by the presence of neutralizing antibodies to the vector in human serum (11).
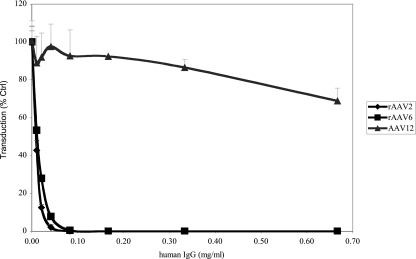
AAV12 was isolated from a simian virus 18 sample obtained from a Vervet monkey. The AAV12 capsid is divergent from that of human AAVs, and therefore, humans may be immunologically naïve to vectors based on AAV12. To test whether human serum has rAAV12-neutralizing activity, we assayed whether IgGs purified from pooled human plasma (IVIG) would block AAV12 transduction in vitro. The neutralization efficiency of IVIG against either rAAV2 or rAAV6 was compared to that against rAAV12 (Fig. 7). In this assay, human rAAV2 and simian rAAV6 displayed similar sensitivity to neutralization by purified pooled IgGs and transduction was inhibited by 50% at a concentration of 0.01 mg/ml. In contrast, rAAV12 was highly resistant to neutralization by pooled human IgGs, even at the highest concentration (0.67 mg/ml), a concentration where 100% inhibition of rAAV2 and rAAV6 was observed; moreover, rAAV12 transduction was reduced by only 30%. Therefore, AAV12 appears to be very resistant to human antibody-mediated neutralization and thus may be useful for in vivo gene therapy applications.
FIG. 7.
rAAV12 is highly resistant to neutralization by human IgGs. rAAV2-GFP, rAAV6-GFP, or rAAV12-GFP was incubated with pooled human IgGs prior to transduction of COS cells. Transduction was analyzed by flow cytometry at 24 h postinoculation. Transduction efficiencies relative to those of an untreated control were plotted. Values are means from three experiments; error bars represent standard deviations.
rAAV12 is serologically distinct from AAV4 and AAV2.
The above data suggest that AAV12 is sufficiently divergent from human AAVs that it is not neutralized by human IVIG. However, AAV12 does have high homology to AAV4, an isolate from African green monkeys, and may not be serologically distinct from other AAVs. We therefore tested the abilities of sera from mice immunized with either AAV4 or AAV2 to inhibit AAV12-mediated gene transfer. No cross-reactivity was observed between isolates, indicating that despite homology between AAV12 and AAV4, they appear to be immunologically distinct isolates (Table 1).
TABLE 1.
Antiserum cross-reactivitya
| Virus | Cross-neutralizing titer for indicated virus
|
||
|---|---|---|---|
| AAV2 | AAV4 | AAV12 | |
| AAV2 | 467 | <1:25 | <1:25 |
| AAV4 | <1:25 | 3,200 | <1:25 |
| AAV12 | <1:25 | <1:25 | 83 |
Cos cells were transduced with a constant amount of rAAV2, rAAV4, or rAAV12 vector expressing GFP (to yield 10 to 100 positive cells/well) and serially diluted serum from a mouse previously infused with a corresponding serotype of rAAV. The number of positive cells detected in the antibody-containing wells was divided by the number of positive cells in the control wells and expressed as a percentage of inhibition against the control. The neutralizing titer of the serum was calculated as the highest dilution that inhibited 50% of the transduction.
rAAV12 transduces salivary glands and skeletal muscles in vivo.
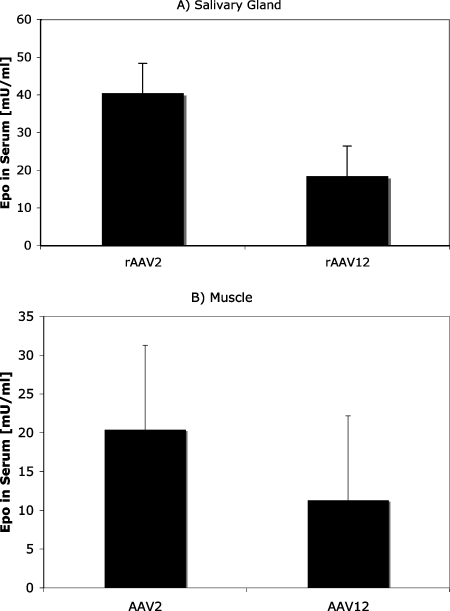
To analyze whether rAAV12 can be utilized as a vector in vivo, we compared the transduction efficiencies of rAAV2- and rAAV12-based vectors expressing erythropoietin in a mouse model. While other serotypes have demonstrated improved transduction activity compared with that of AAV2, the in vivo activity of AAV2 has been well studied by many groups and is often used as a standard when testing the transduction activity of a new vector in vivo. Salivary glands, easily accessible natural secretory organs, are a potent depot organ for the production of proteins either in the blood or in saliva (6). Submandibular glands of BALB/c mice were cannulated and injected with 1010 particles of rAAV2-Epo or rAAV12-Epo. While muscle is not normally considered a secretory tissue like salivary glands, it has served as a depot organ for the expression of several recombinant proteins, including blood coagulation factors (7). Transduction with rAA12-Epo in either muscle or salivary glands resulted in similar erythropoietin levels in blood 8 weeks after transduction with rAAV12, demonstrating that rAAV12 can be utilized as a vector for in vivo gene transfer applications (Fig. 8).
FIG. 8.
rAAV12 transduces salivary glands and skeletal muscles in vivo. Male BALB/c mice were administered 109 particles of either AAV2-hEPO or AAV12-hEPO by retrograde ductal delivery to either the submandibular glands or the tibialis anterior (two injection sites per muscle). Secretion of hEPO in mouse serum was determined 4 weeks after transduction by an enzyme-linked immunosorbent assay. Error bars represent standard deviations (n = 4).
DISCUSSION
rAAV is an attractive vector system for gene therapy. The wild-type virus is considered nonpathogenic, and vectors based on AAV have a broad tropism, transduce dividing and nondividing cells, and mediate long-term expression in vivo (11). While all AAV serotypes share a common genome organization and capsid structure, they can have unique cell tropisms as a result of binding different cellular factors. Expressions of cell surface HSPGs have been identified as critical determinants for AAV2 and AAV3 transduction, whereas sialic acid mediates AAV4, AAV5, AAV6, and BAAV attachment to the cell (12, 21, 23, 24, 28, 30).
We have identified and characterized AAV12, the only AAV described so far that does not depend on either heparan sulfate or sialic acid for transduction. Initial experiments demonstrated that rAAV12-mediated gene transfer requires membrane-associated proteins, unlike BAAV, which depends on glycolipids (21). Further studies will be required to identify the attachment factor and receptor for AAV12. The unique virus-cell interaction results in a unique cell tropism. Lung-derived A549 cells and the colon cell line HCT15 were not permissive for rAAV4, which shares 78% capsid homology with AAV12, but could be transduced with rAAV12. While the initial virus-cell binding and uptake are atypical for an AAV, the mechanism of virus intracellular trafficking appears similar to that of AAV2. Our studies show that acidification of an endosomal compartment is required for transduction.
AAV12 has unique immunological properties. rAAV12 was highly resistant to neutralization by circulating antibodies from human serum and is immunologically distinct from both AAV4 and AAV2. Regions in the AAV12 capsid, which are not well conserved compared to those of other primate AAVs, could play a major role in the neutralization resistance, and even minor sequence changes have been reported to effect antibody neutralization activity (9). Further studies of the immunological properties will be necessary to help define the exact epitopes that are recognized by human neutralizing antibodies. The unusual virus-cell interaction of rAAV12, together with a high resistance to neutralization by human serum, renders rAAV12 an interesting candidate for gene transfer applications. The feasibility of rAAV12 as a vector was demonstrated in a mouse model. Muscle and salivary glands were transduced, demonstrating the utility of AAV12 as a vector for gene transfer in vivo; however, a more extensive analysis of different tissue types will be required to identify a unique application for AAV12 compared with those for other AAV vectors.
The distinct transduction pathway for this isolate compared to those for other AAVs highlights the individuality of this isolate and suggests that identification of its receptor and coreceptor will be important in understanding the capsid structures necessary for parvovirus infection. Furthermore, receptor identification will allow for targeting of AAV12 to a specific gene transfer application in which its unique transduction activity can be fully utilized.
Acknowledgments
We thank Kathleen Bolland and the NIDCR Sequencing Core facility for their excellent support and the NIH Fellows Editorial Board for review of the manuscript.
This research was supported by the NIDCR Intramural Research Program of the NIH.
Footnotes
Published ahead of print on 28 November 2007.
REFERENCES
- 1.Arella, M., S. Garzon, J. Bergeron, and P. Tijssen. 1990. Handbook of parvoviruses, vol. 1. CRC Press, Boca Raton, FL.
- 2.Atchison, R. W., B. C. Casto, and W. M. Hammon. 1965. Adeno-associated defective virus particles. Science 1149754-755. [DOI] [PubMed] [Google Scholar]
- 3.Baccaglini, L., A. T. Shamsul Hoque, R. B. Wellner, C. M. Goldsmith, R. S. Redman, V. Sankar, A. Kingman, K. M. Barnhart, C. J. Wheeler, and B. J. Baum. 2001. Cationic liposome-mediated gene transfer to rat salivary epithelial cells in vitro and in vivo. J. Gene Med. 382-90. [DOI] [PubMed] [Google Scholar]
- 4.Bartlett, J. S., R. Wilcher, and R. J. Samulski. 2000. Infectious entry pathway of adeno-associated virus and adeno-associated virus vectors. J. Virol. 742777-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baum, B. J. 2002. Biomedical research, oral medicine, and the future. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 94141-142. [DOI] [PubMed] [Google Scholar]
- 6.Baum, B. J., J. C. Atkinson, L. Baccaglini, M. E. Berkman, J. S. Brahim, C. Davis, H. E. Lancaster, Y. Marmary, A. C. O'Connell, B. C. O'Connell, S. Wang, Y. Xu, H. Yamagishi, and P. C. Fox. 1998. The mouth is a gateway to the body: gene therapy in 21st-century dental practice. J. Calif. Dent. Assoc. 26455-460. [PubMed] [Google Scholar]
- 7.Chao, H., Y. Liu, J. Rabinowitz, C. Li, R. J. Samulski, and C. E. Walsh. 2000. Several log increase in therapeutic transgene delivery by distinct adeno-associated viral serotype vectors. Mol. Ther. 2619-623. [DOI] [PubMed] [Google Scholar]
- 8.Davidson, B. L., C. S. Stein, J. A. Heth, I. Martins, R. M. Kotin, T. A. Derksen, J. Zabner, A. Ghodsi, and J. A. Chiorini. 2000. Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proc. Natl. Acad. Sci. USA 973428-3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao, G., M. R. Alvira, S. Somanathan, Y. Lu, L. H. Vandenberghe, J. J. Rux, R. Calcedo, J. Sanmiguel, Z. Abbas, and J. M. Wilson. 2003. Adeno-associated viruses undergo substantial evolution in primates during natural infections. Proc. Natl. Acad. Sci. USA 1006081-6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Georg-Fries, B., S. Biederlack, J. Wolf, and H. zur Hausen. 1984. Analysis of proteins, helper dependence, and seroepidemiology of a new human parvovirus. Virology 13464-71. [DOI] [PubMed] [Google Scholar]
- 11.Grimm, D., and M. A. Kay. 2003. From virus evolution to vector revolution: use of naturally occurring serotypes of adeno-associated virus (AAV) as novel vectors for human gene therapy. Curr. Gene Ther. 3281-304. [DOI] [PubMed] [Google Scholar]
- 12.Kaludov, N., K. E. Brown, R. W. Walters, J. Zabner, and J. A. Chiorini. 2001. Adeno-associated virus serotype 4 (AAV4) and AAV5 both require sialic acid binding for hemagglutination and efficient transduction but differ in sialic acid linkage specificity. J. Virol. 756884-6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaludov, N., B. Handelman, and J. A. Chiorini. 2002. Scalable purification of adeno-associated virus type 2, 4, or 5 using ion-exchange chromatography. Hum. Gene Ther. 131235-1243. [DOI] [PubMed] [Google Scholar]
- 14.Katano, H., S. Afione, M. Schmidt, and J. A. Chiorini. 2004. Identification of adeno-associated virus contamination in cell and virus stocks by PCR. BioTechniques 36676-680. [DOI] [PubMed] [Google Scholar]
- 15.Katen, L. J., M. M. Januszeski, W. F. Anderson, K. J. Hasenkrug, and L. H. Evans. 2001. Infectious entry by amphotropic as well as ecotropic murine leukemia viruses occurs through an endocytic pathway. J. Virol. 755018-5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kovacs, P., M. Pinter, and G. Csaba. 2000. Effect of glucosphingolipid synthesis inhibitor (PPMP and PDMP) treatment on Tetrahymena pyriformis: data on the evolution of the signaling system. Cell Biochem. Funct. 18269-280. [DOI] [PubMed] [Google Scholar]
- 17.McPherson, R. A., L. J. Rosenthal, and J. A. Rose. 1985. Human cytomegalovirus completely helps adeno-associated virus replication. Virology 147217-222. [DOI] [PubMed] [Google Scholar]
- 18.Ogston, P., K. Raj, and P. Beard. 2000. Productive replication of adeno-associated virus can occur in human papillomavirus type 16 (HPV-16) episome-containing keratinocytes and is augmented by the HPV-16 E2 protein. J. Virol. 743494-3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parks, W. P., J. L. Melnick, R. Rongey, and H. D. Mayor. 1967. Physical assay and growth cycle studies of a defective adeno-satellite virus. J. Virol. 1171-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlehofer, J. R., M. Ehrbar, and H. zur Hausen. 1986. Vaccinia virus, herpes simplex virus, and carcinogens induce DNA amplification in a human cell line and support replication of a helpervirus dependent parvovirus. Virology 152110-117. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt, M., and J. A. Chiorini. 2006. Gangliosides are essential for bovine adeno-associated virus entry. J. Virol. 805516-5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt, M., E. Grot, P. Cervenka, S. Wainer, C. Buck, and J. A. Chiorini. 2006. Identification and characterization of novel adeno-associated virus isolates in ATCC virus stocks. J. Virol. 805082-5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt, M., H. Katano, I. Bossis, and J. A. Chiorini. 2004. Cloning and characterization of a bovine adeno-associated virus. J. Virol. 786509-6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seiler, M. P., A. D. Miller, J. Zabner, and C. L. Halbert. 2006. Adeno-associated virus types 5 and 6 use distinct receptors for cell entry. Hum. Gene Ther. 1710-19. [DOI] [PubMed] [Google Scholar]
- 25.Smith, R. H., S. A. Afione, and R. M. Kotin. 2002. Transposase-mediated construction of an integrated adeno-associated virus type 5 helper plasmid. BioTechniques 33204-206, 208, 210-211. [DOI] [PubMed] [Google Scholar]
- 26.Summerford, C., and R. J. Samulski. 1998. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J. Virol. 721438-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voutetakis, A., M. R. Kok, C. Zheng, I. Bossis, J. Wang, A. P. Cotrim, N. Marracino, C. M. Goldsmith, J. A. Chiorini, Y. P. Loh, L. K. Nieman, and B. J. Baum. 2004. Reengineered salivary glands are stable endogenous bioreactors for systemic gene therapeutics. Proc. Natl. Acad. Sci. USA 1013053-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walters, R. W., S. M. Yi, S. Keshavjee, K. E. Brown, M. J. Welsh, J. A. Chiorini, and J. Zabner. 2001. Binding of adeno-associated virus type 5 to 2,3-linked sialic acid is required for gene transfer. J. Biol. Chem. 27620610-20616. [DOI] [PubMed] [Google Scholar]
- 29.Walz, C., A. Deprez, T. Dupressoir, M. Durst, M. Rabreau, and J. R. Schlehofer. 1997. Interaction of human papillomavirus type 16 and adeno-associated virus type 2 co-infecting human cervical epithelium. J. Gen. Virol. 781441-1452. [DOI] [PubMed] [Google Scholar]
- 30.Wu, Z., E. Miller, M. Agbandje-McKenna, and R. J. Samulski. 2006. α2,3 and α2,6 N-linked sialic acids facilitate efficient binding and transduction by adeno-associated virus types 1 and 6. J. Virol. 809093-9103. [DOI] [PMC free article] [PubMed] [Google Scholar]