Abstract
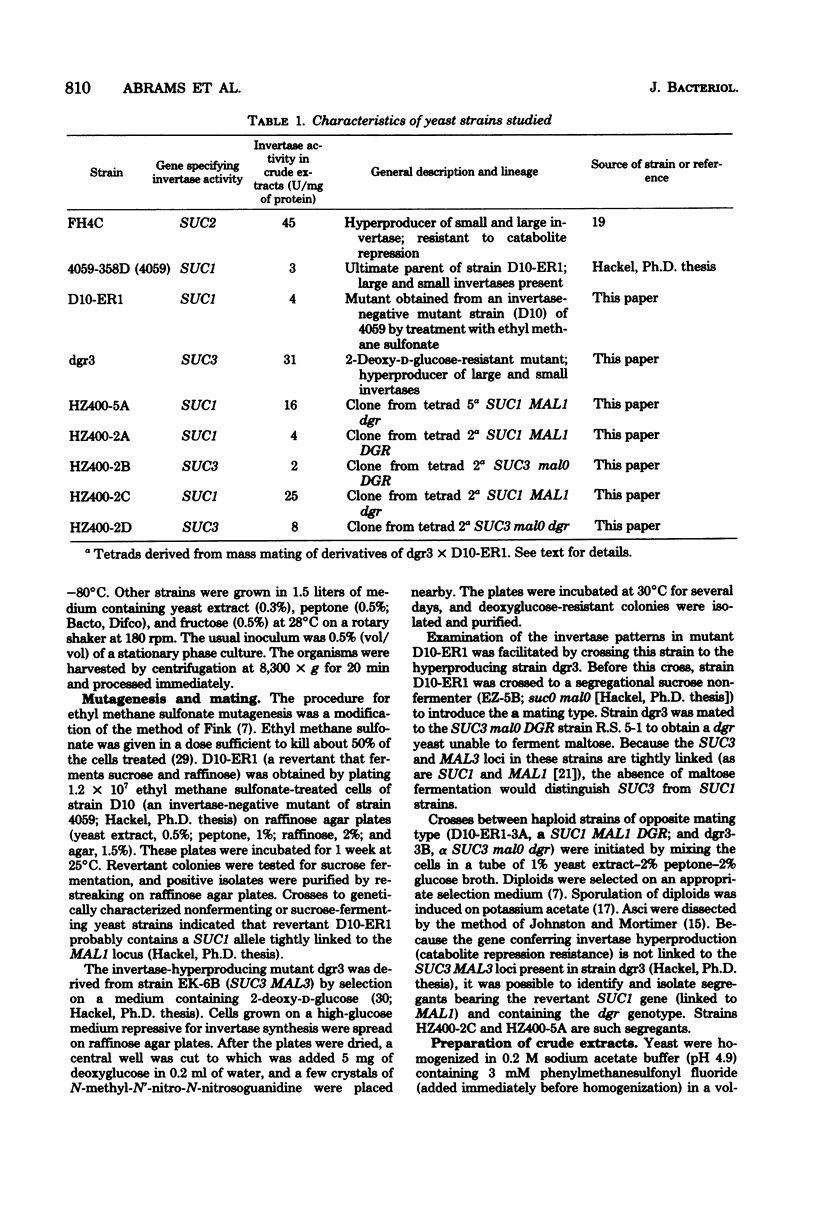
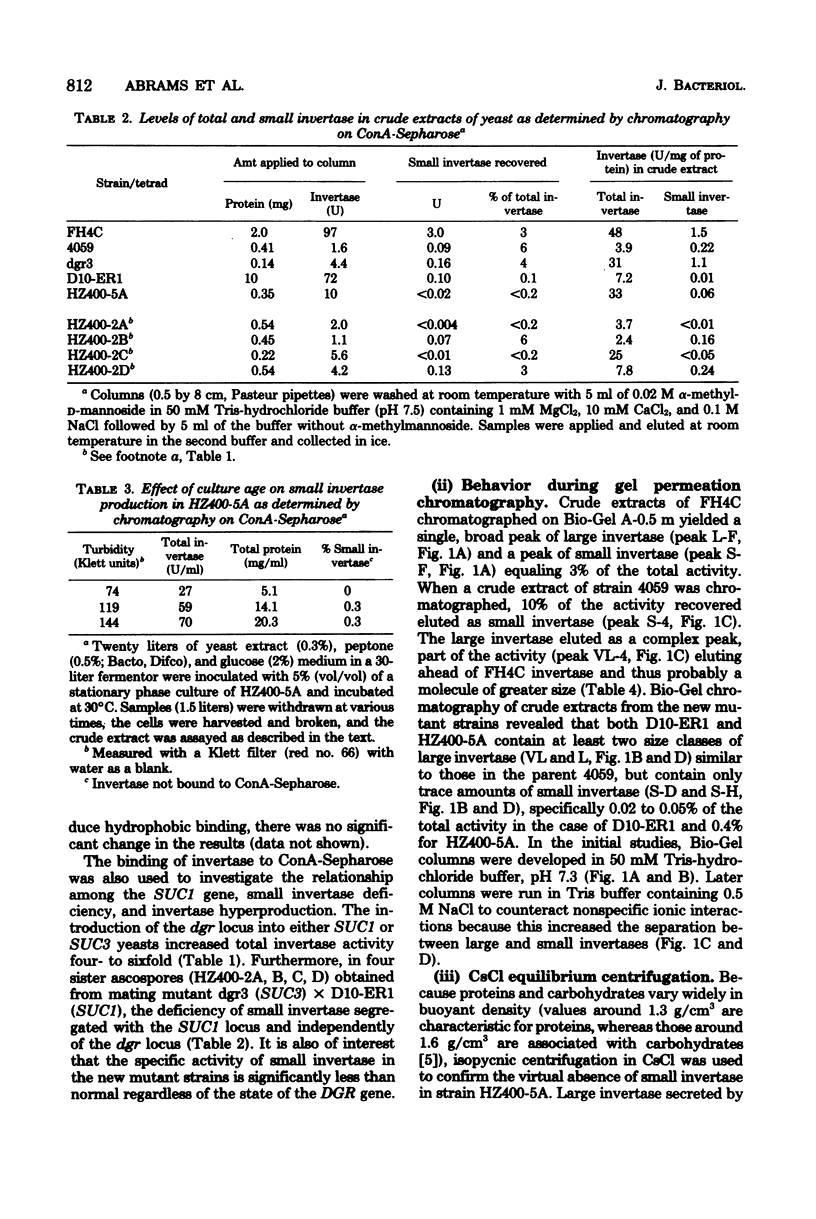
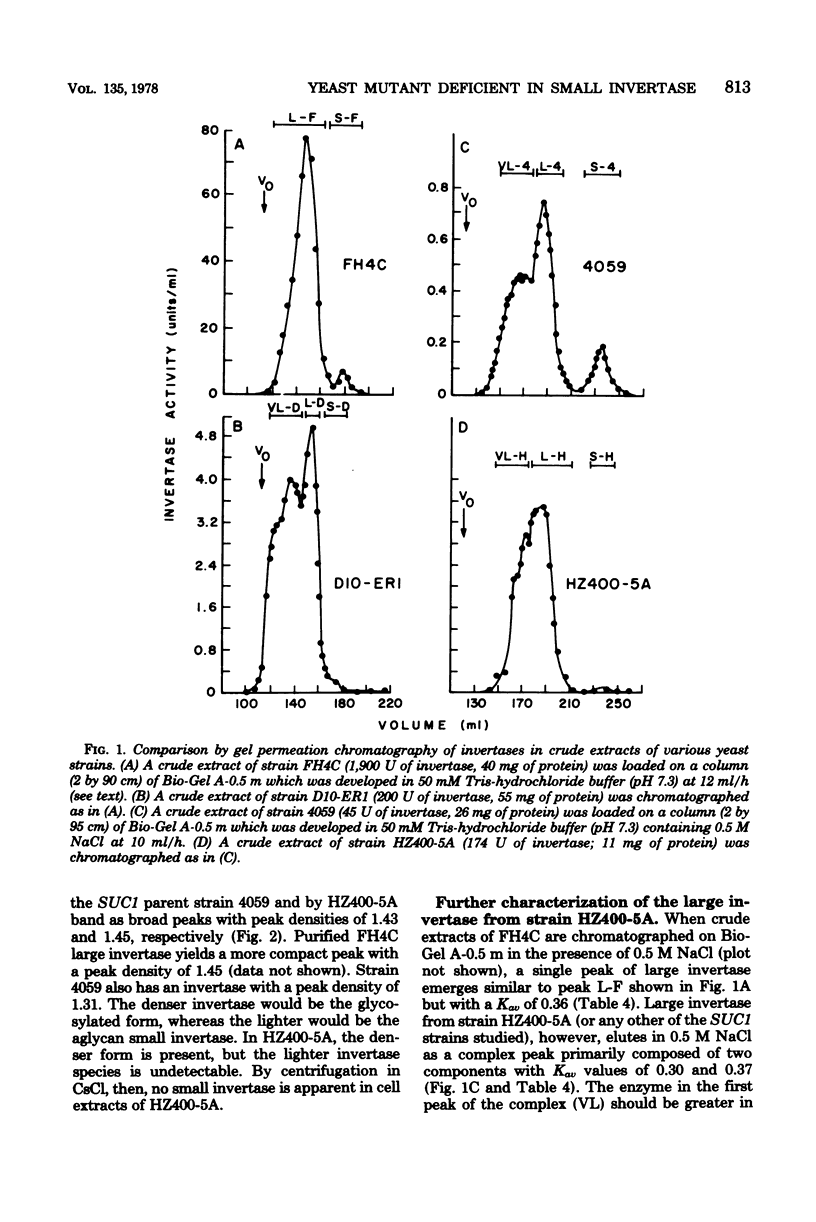
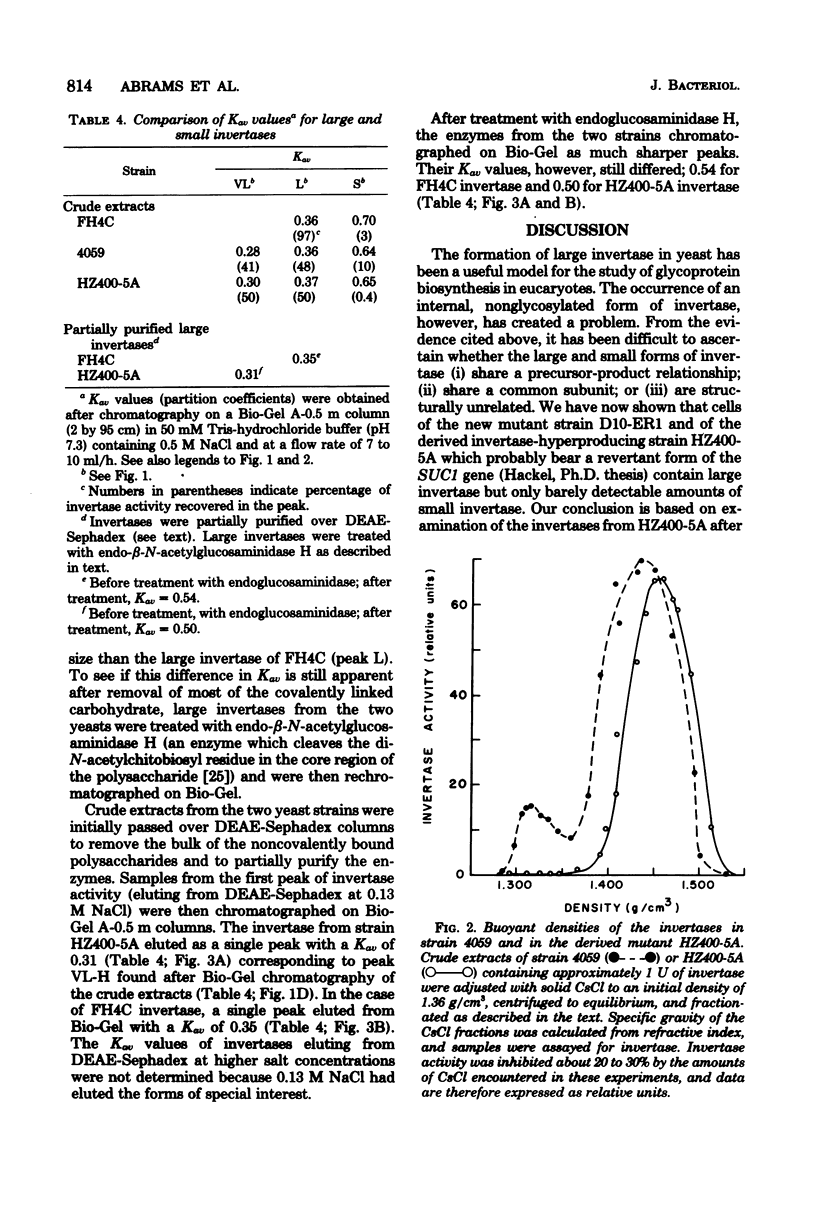
A mutant strain of Saccharomyces cerevisiae (D10-ER1) has been isolated after a two-step mutagenesis of strain 4059-358D (SUC 1) using ethyl methane sulfonate. Cells of this new strain produced a level of total invertase equaling that of 4059 but contained only trace amounts of the small, internal, aglycan form of the enzyme (less than 0.1% of total in D10-ER1 compared with 6% in 4059). When D10.ER1 was crossed with an invertase-hyperproducing strain dgr3 (SUC3), progeny were isolated (HZ400-5A and HZ400-2C) in which levels of total invertase had at least quadrupled. The percentage of small invertase, however, remained insignificant. Levels of small invertase in strain HZ400-5A were determined by affinity chromatography on conconavalin A-Sepharose, gel permeation chromatography, and isopycnic centrifugation in CsCl. The large invertase of the SUC1 yeasts described here was found to contain a form apparently greater in size than the large invertase of the SUC2 strain FH4C; this probably reflects a higher content of carbohydrate. The overall results of this study do not support a direct structural relationship between large and small invertases. The implications on invertase biosynthesis and structure are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baseer A., Shall S. Properties of the internal invertase of yeast, Saccharomyces cerevisiae. Biochim Biophys Acta. 1971 Oct;250(1):192–202. doi: 10.1016/0005-2744(71)90133-1. [DOI] [PubMed] [Google Scholar]
- Creeth J. M., Denborough M. A. The use of equilibrium-density-gradient methods for the preparation and characterization of blood-group-specific glycoproteins. Biochem J. 1970 May;117(5):879–891. doi: 10.1042/bj1170879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE LA FUENTE G., SOLS A. Transport of sugars in yeasts. II. Mechanisms of utilization of disaccharides and related glycosides. Biochim Biophys Acta. 1962 Jan 1;56:49–62. doi: 10.1016/0006-3002(62)90526-7. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN I. J., HOLLERMAN C. E., SMITH E. E. PROTEIN-CARBOHYDRATE INTERACTION. II. INHIBITION STUDIES ON THE INTERACTION OF CONCANAVALIN A WITH POLYSACCHARIDES. Biochemistry. 1965 May;4:876–883. doi: 10.1021/bi00881a013. [DOI] [PubMed] [Google Scholar]
- Gallili G., Lampen J. O. Large and small invertases and the yeast cell cycle. Pattern of synthesis and sensitivity to tunicamycin. Biochim Biophys Acta. 1977 Mar 2;475(1):113–122. doi: 10.1016/0005-2787(77)90345-8. [DOI] [PubMed] [Google Scholar]
- Gascón S., Lampen J. O. Purification of the internal invertase of yeast. J Biol Chem. 1968 Apr 10;243(7):1567–1572. [PubMed] [Google Scholar]
- Gascón S., Neumann N. P., Lampen J. O. Comparative study of the properties of the purified internal and external invertases from yeast. J Biol Chem. 1968 Apr 10;243(7):1573–1577. [PubMed] [Google Scholar]
- Gascón S., Ottolenghi P. Invertase isozymes and their localization in yeast. C R Trav Lab Carlsberg. 1967;36(5):85–93. [PubMed] [Google Scholar]
- Goldstein A., Lampen J. O. Beta-D-fructofuranoside fructohydrolase from yeast. Methods Enzymol. 1975;42:504–511. doi: 10.1016/0076-6879(75)42159-0. [DOI] [PubMed] [Google Scholar]
- Hackel R. A. Genetic control of invertase formation in Saccharomyces cerevisiae. I. Isolation and characterization of mutants affecting sucrose utilization. Mol Gen Genet. 1975 Oct 22;140(4):361–370. doi: 10.1007/BF00267327. [DOI] [PubMed] [Google Scholar]
- JOHNSTON J. R., MORTIMER R. K. Use of snail digestive juice in isolation of yeast spore tetrads. J Bacteriol. 1959 Aug;78:292–292. doi: 10.1128/jb.78.2.292-292.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- McClary D. O., Nulty W. L., Miller G. R. EFFECT OF POTASSIUM VERSUS SODIUM IN THE SPORULATION OF SACCHAROMYCES. J Bacteriol. 1959 Sep;78(3):362–368. doi: 10.1128/jb.78.3.362-368.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenecourt B. S., Kuo S. C., Lampen J. O. Saccharomyces mutants with invertase formation resistant to repression by hexoses. J Bacteriol. 1973 Apr;114(1):233–238. doi: 10.1128/jb.114.1.233-238.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer R. K., Hawthorne D. C. Genetic mapping in Saccharomyces. Genetics. 1966 Jan;53(1):165–173. doi: 10.1093/genetics/53.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann N. P., Lampen J. O. Purification and properties of yeast invertase. Biochemistry. 1967 Feb;6(2):468–475. doi: 10.1021/bi00854a015. [DOI] [PubMed] [Google Scholar]
- Ottolenghi P. Some properties of five non-allelic -D-fructofuranosidases (invertases) of Saccharomyces. C R Trav Lab Carlsberg. 1971;38(13):213–221. [PubMed] [Google Scholar]
- Tarentino A. L., Maley F. Purification and properties of an endo-beta-N-acetylglucosaminidase from Streptomyces griseus. J Biol Chem. 1974 Feb 10;249(3):811–817. [PubMed] [Google Scholar]
- Trimble R. B., Maley F. Subunit structure of external invertase from Saccharomyces cerevisiae. J Biol Chem. 1977 Jun 25;252(12):4409–4412. [PubMed] [Google Scholar]
- Waheed A., Shall S. Chemical modification of the active site of yeast invertase. Biochim Biophys Acta. 1971 Jul 21;242(1):172–189. doi: 10.1016/0005-2744(71)90097-0. [DOI] [PubMed] [Google Scholar]
- Zimmermann F. K., Khan N. A., Eaton N. R. Identification of new genes involved in disaccharide fermentation in yeast. Mol Gen Genet. 1973;123(1):29–41. doi: 10.1007/BF00282986. [DOI] [PubMed] [Google Scholar]
- Zimmermann F. K., Scheel I. Mutants of Saccharomyces cerevisiae resistant to carbon catabolite repression. Mol Gen Genet. 1977 Jul 7;154(1):75–82. doi: 10.1007/BF00265579. [DOI] [PubMed] [Google Scholar]


