Abstract
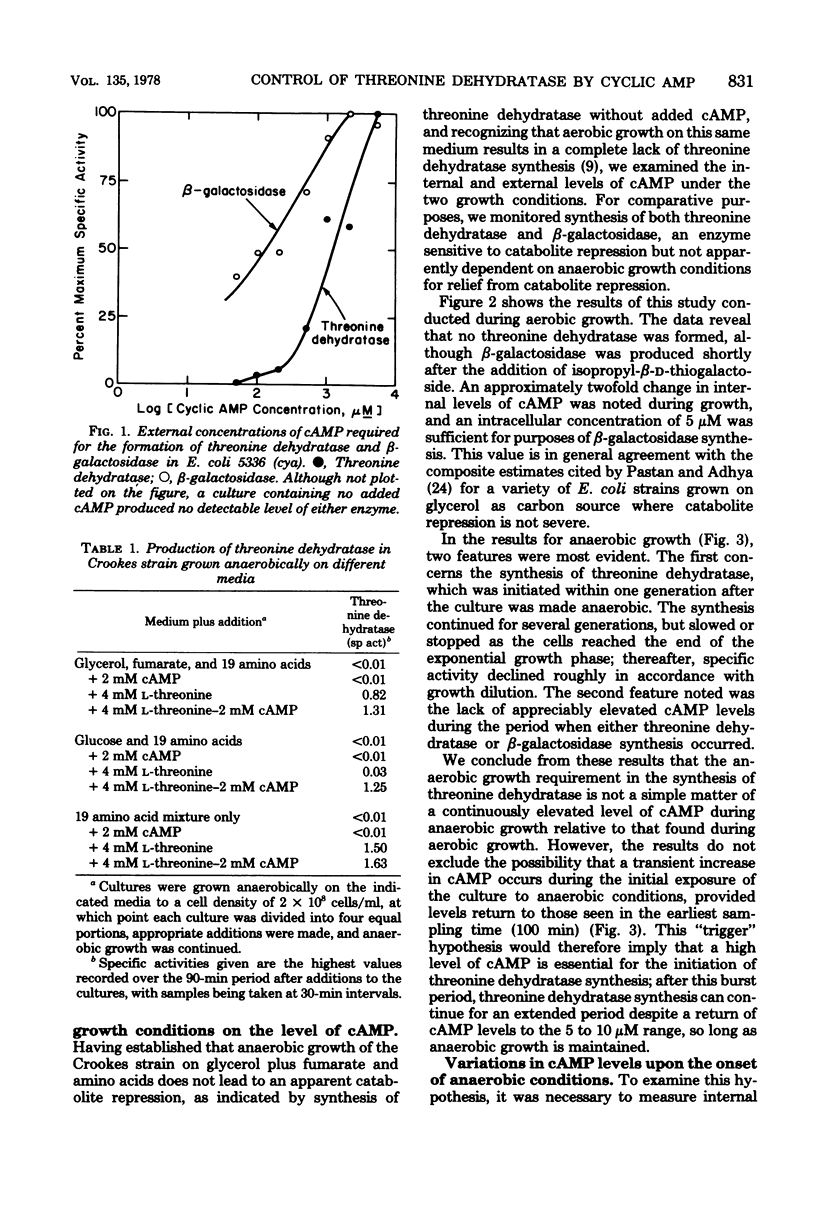
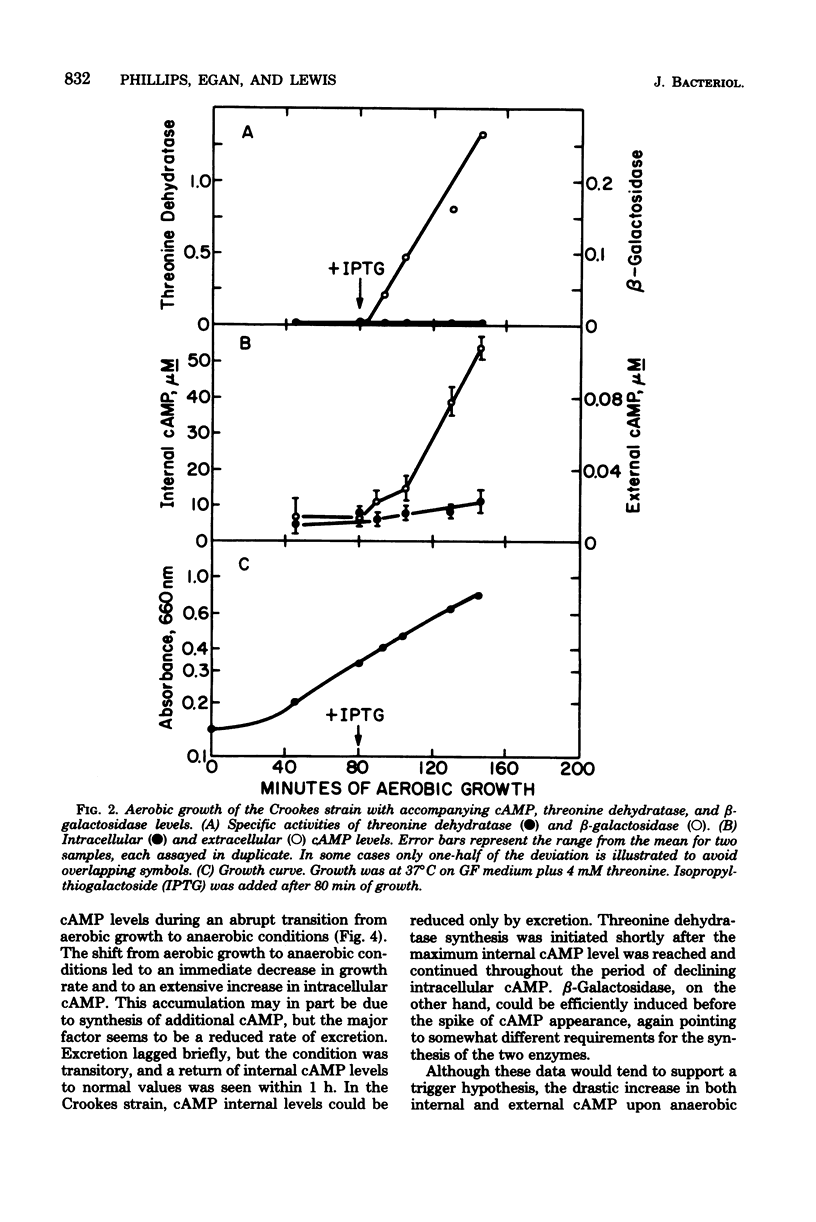
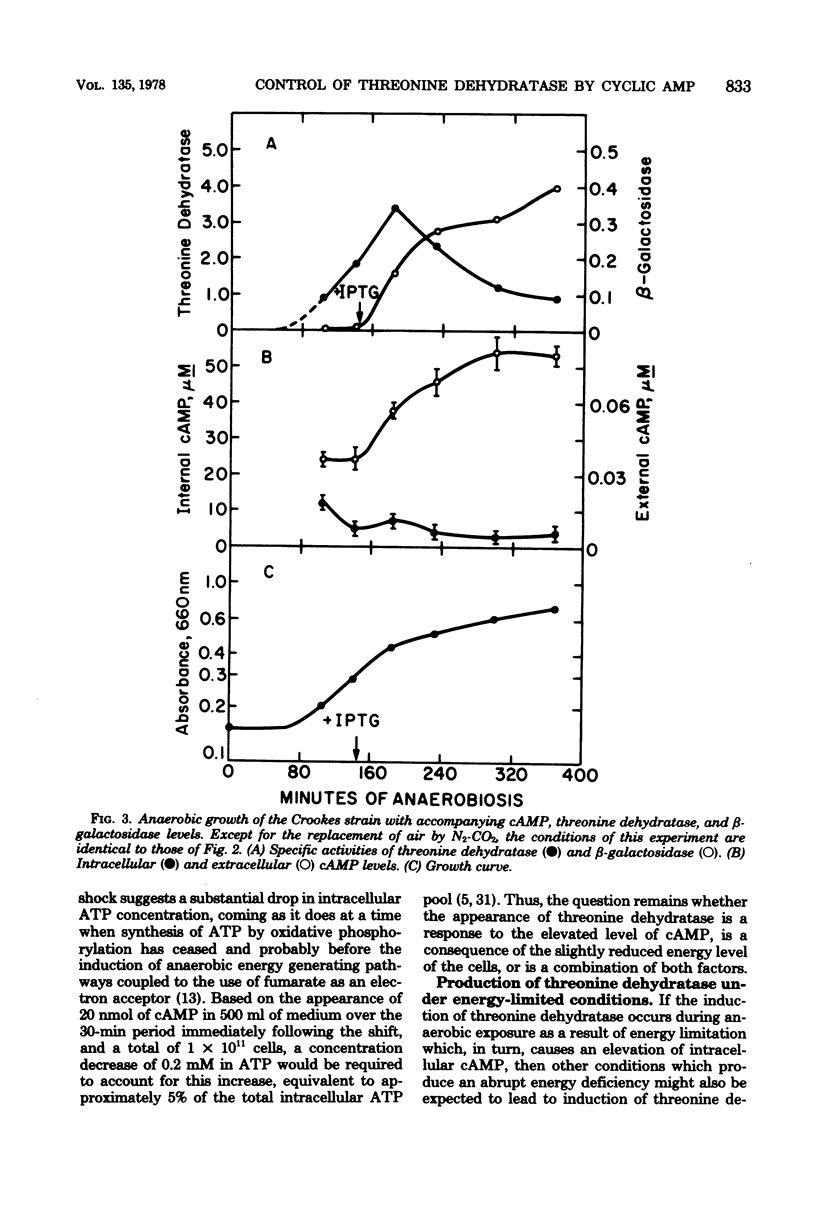
To explain the requirement for anaerobic conditions in the induction of biodegradative L-threonine dehydratase in Escherichia coli, Crookes strain, measurements of cyclic AMP (cAMP) were made during aerobic and anaerobic growth and upon an aerobic-to-anaerobic transition. Internal cAMP levels were similar (5 to 10 muM) throughout exponential growth, whether aerobic or anaerobic, but only during anaerobiosis was threonine dehydratase synthesized. When an exponentially growing aerobic culture was made anaerobic, a sharp increase in internal cAMP was noted, reaching 300 muM within 10 min and declining thereafter to normal anaerobic levels. Threonine dehydratase synthesis was detected immediately after the attainment of peak cAMP levels and continued for several generations. A similar pattern but with less accumulation of cAMP and less threonine dehydratase production was also noted upon treatment of an aerobically growing culture with KCN. Pyruvate addition at the time of anaerobic shock severely affected both cAMP accumulation and threonine dehydratase synthesis; however, externally added cAMP could partially counter the pyruvate effect on enzyme synthesis. The conclusion was reached that conditions which resulted in a temporary energy deficit brought about the major accumulation of cAMP, and this elevated level served as a signal for initiation of threonine dehydratase synthesis to supply energy by the nonoxidative degradation of threonine.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen K. B., von Meyenburg K. Charges of nicotinamide adenine nucleotides and adenylate energy charge as regulatory parameters of the metabolism in Escherichia coli. J Biol Chem. 1977 Jun 25;252(12):4151–4156. [PubMed] [Google Scholar]
- Bragg P. D., Davies P. L., Hou C. Function of energy-dependent transhydrogenase in Escherichia coli. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1248–1255. doi: 10.1016/0006-291x(72)90969-2. [DOI] [PubMed] [Google Scholar]
- Buettner M. J., Spitz E., Rickenberg H. V. Cyclic adenosine 3',5'-monophosphate in Escherichia coli. J Bacteriol. 1973 Jun;114(3):1068–1073. doi: 10.1128/jb.114.3.1068-1073.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascieri M., Amann R. P., Hammerstedt R. H. Adenine nucleotide changes at initiation of bull sperm motility. J Biol Chem. 1976 Feb 10;251(3):787–793. [PubMed] [Google Scholar]
- Chapman A. G., Fall L., Atkinson D. E. Adenylate energy charge in Escherichia coli during growth and starvation. J Bacteriol. 1971 Dec;108(3):1072–1086. doi: 10.1128/jb.108.3.1072-1086.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOBROGOSZ W. J. THE INFLUENCE OF NITRATE AND NITRITE REDUCTION ON CATABOLITE REPRESSION IN ESCHERICHIA COLI. Biochim Biophys Acta. 1965 May 4;100:553–566. doi: 10.1016/0304-4165(65)90025-5. [DOI] [PubMed] [Google Scholar]
- Dills S. E., Dobrogosz W. J. Cyclic adenosine 3',5'-monophosphate regulation of membrane energetics in Escherichia coli. J Bacteriol. 1977 Sep;131(3):854–865. doi: 10.1128/jb.131.3.854-865.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrogosz W. J. Altered end-product patterns and catabolite repression in Escherichia coli. J Bacteriol. 1966 Jun;91(6):2263–2269. doi: 10.1128/jb.91.6.2263-2269.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan R. M., Phillips A. T. Requirements for induction of the biodegradative threonine dehydratase in Escherichia coli. J Bacteriol. 1977 Nov;132(2):370–376. doi: 10.1128/jb.132.2.370-376.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W., Rothman-Denes L. B., Hesse J. Adenosine 3':5'-cyclic monophosphate as mediator of catabolite repression in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2300–2304. doi: 10.1073/pnas.72.6.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke U. Tritosol: a new scintillation cocktail based on Triton X-100. Anal Biochem. 1975 Feb;63(2):555–558. doi: 10.1016/0003-2697(75)90379-6. [DOI] [PubMed] [Google Scholar]
- Greene R., Magasanik B. The mode of action of levallorphan as an inhibitor of cell growth. Mol Pharmacol. 1967 Sep;3(5):453–472. [PubMed] [Google Scholar]
- Haddock B. A., Jones C. W. Bacterial respiration. Bacteriol Rev. 1977 Mar;41(1):47–99. doi: 10.1128/br.41.1.47-99.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAKMAN R. S., SUTHERLAND E. W. ADENOSINE 3',5'-PHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1965 Mar;240:1309–1314. [PubMed] [Google Scholar]
- Okinaka R. T., Dobrogosz W. J. Catabolite repression and pyruvate metabolism in Escherichia coli. J Bacteriol. 1967 May;93(5):1644–1650. doi: 10.1128/jb.93.5.1644-1650.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pall M. L. Cyclic AMP and the plasma membrane potential in Neurospora crassa. J Biol Chem. 1977 Oct 25;252(20):7146–7150. [PubMed] [Google Scholar]
- Pastan I., Adhya S. Cyclic adenosine 5'-monophosphate in Escherichia coli. Bacteriol Rev. 1976 Sep;40(3):527–551. doi: 10.1128/br.40.3.527-551.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman R. L., Pastan I. Pleiotropic deficiency of carbohydrate utilization in an adenyl cyclase deficient mutant of Escherichia coli. Biochem Biophys Res Commun. 1969 Sep 24;37(1):151–157. doi: 10.1016/0006-291x(69)90893-6. [DOI] [PubMed] [Google Scholar]
- Phillips A. T., Wood W. A. The mechanism of action of 5'-adenylic acid-activated threonine dehydrase. J Biol Chem. 1965 Dec;240(12):4703–4709. [PubMed] [Google Scholar]
- Potter R., Kapoor V., Newman E. B. Role of threonine dehydrogenase in Escherichia coli threonine degradation. J Bacteriol. 1977 Nov;132(2):385–391. doi: 10.1128/jb.132.2.385-391.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickenberg H. V. Cyclic AMP in prokaryotes. Annu Rev Microbiol. 1974;28(0):353–369. doi: 10.1146/annurev.mi.28.100174.002033. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U., McCaman M. T. Regulation of intracellular adenosine cyclic 3':5'-monophosphate levels in Escherichia coli and Salmonella typhimurium. Evidence for energy-dependent excretion of the cyclic nucleotide. J Biol Chem. 1975 Oct 10;250(19):7593–7601. [PubMed] [Google Scholar]
- Shizuta Y., Hayaishi O. Regulation of biodegradative threonine deaminase synthesis in Escherichia coli by cyclic adenosine 3',5'-monophosphate. J Biol Chem. 1970 Oct 25;245(20):5416–5423. [PubMed] [Google Scholar]
- Swedes J. S., Sedo R. J., Atkinson D. E. Relation of growth and protein synthesis to the adenylate energy charge in an adenine-requiring mutant of Escherichia coli. J Biol Chem. 1975 Sep 10;250(17):6930–6938. [PubMed] [Google Scholar]
- UMBARGER H. E., BROWN B. Threonine deamination in Escherichia coli. II. Evidence for two L-threonine deaminases. J Bacteriol. 1957 Jan;73(1):105–112. doi: 10.1128/jb.73.1.105-112.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD W. A., GUNSALUS I. C. Serine and threonine desaminaes of Escherichia coli; activators for a cell-free enzyme. J Biol Chem. 1949 Nov;181(1):171–182. [PubMed] [Google Scholar]
- Yui Y., Watanabe Y., Ito S., Shizuta Y., Hayaishi O. Multivalent induction of biodegradative threonine deaminase. J Bacteriol. 1977 Nov;132(2):363–369. doi: 10.1128/jb.132.2.363-369.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


