Abstract
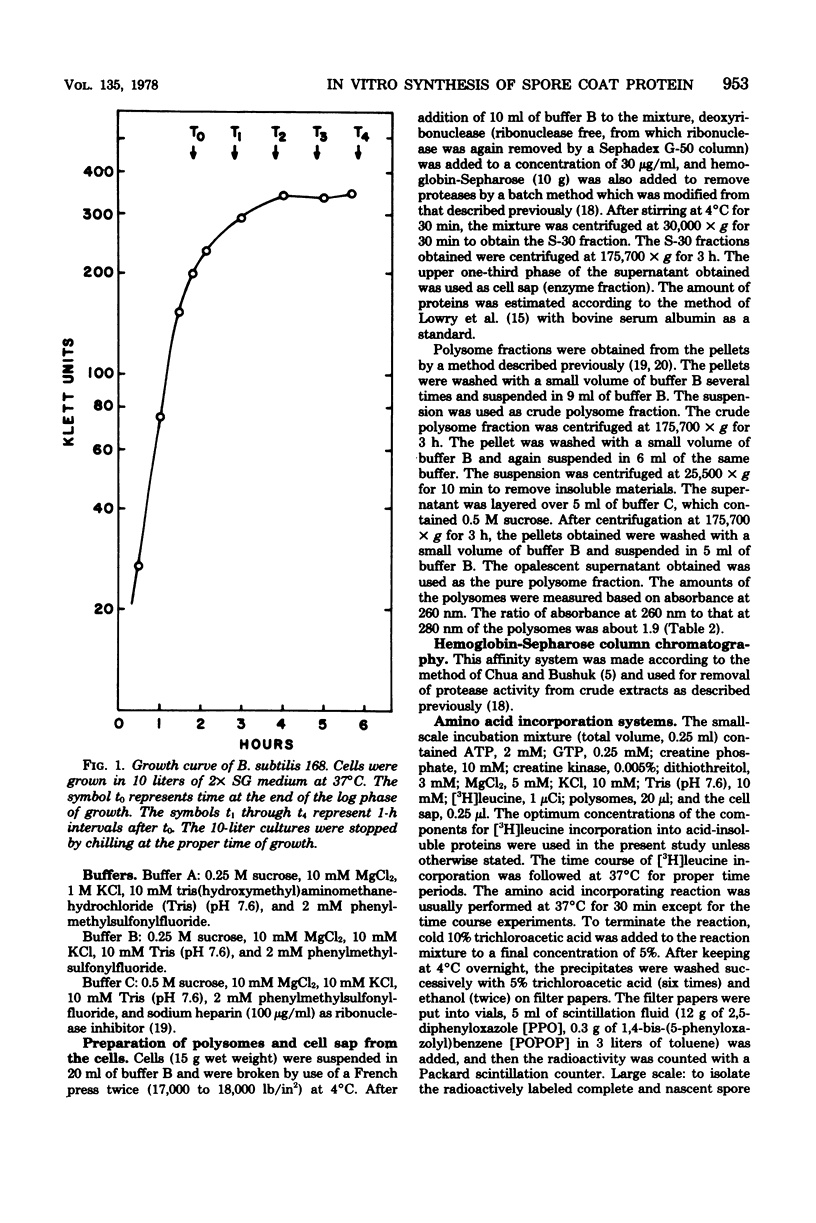
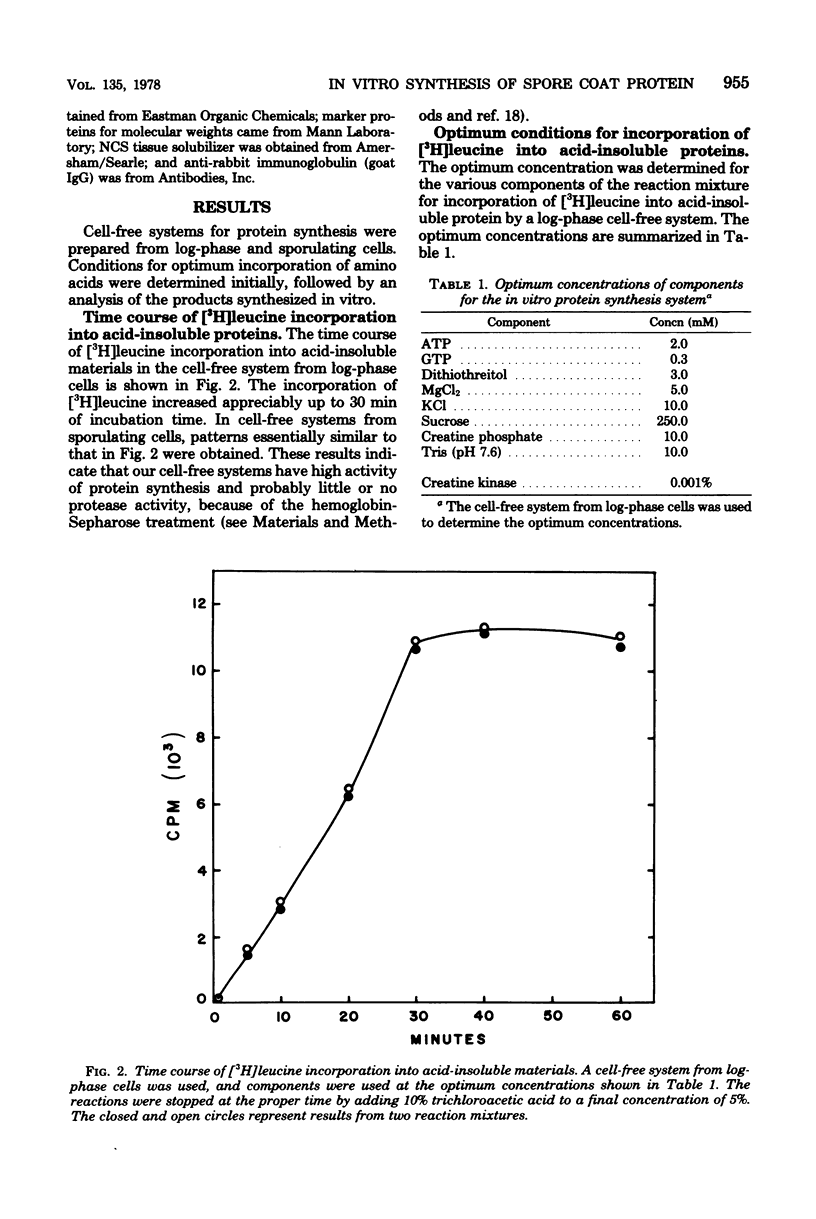
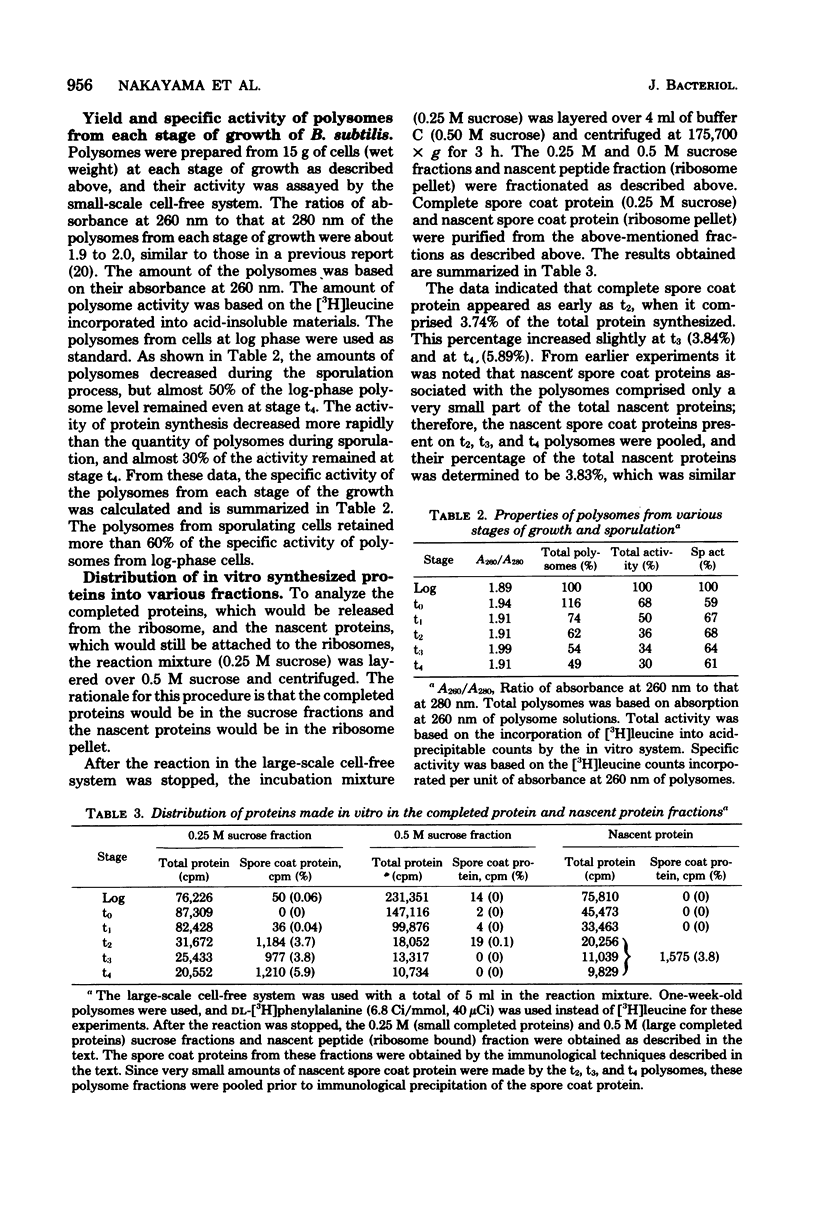
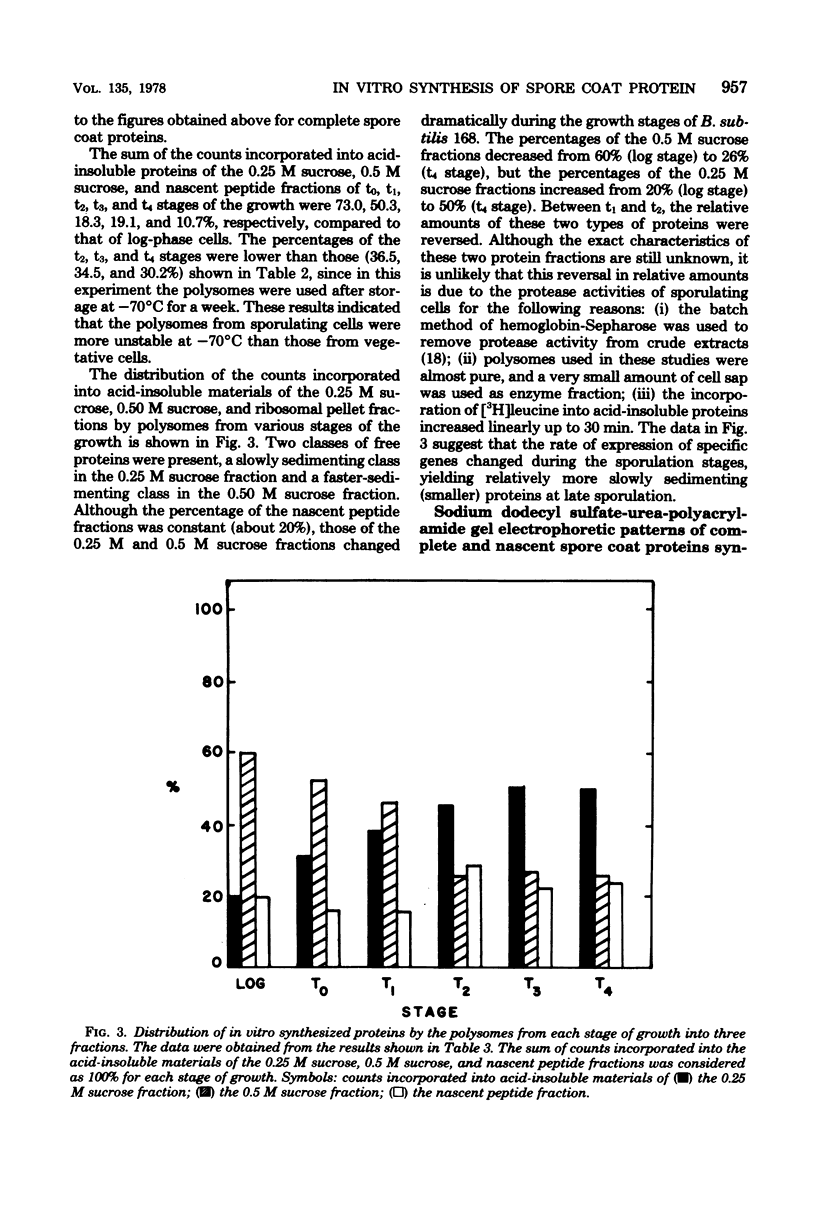
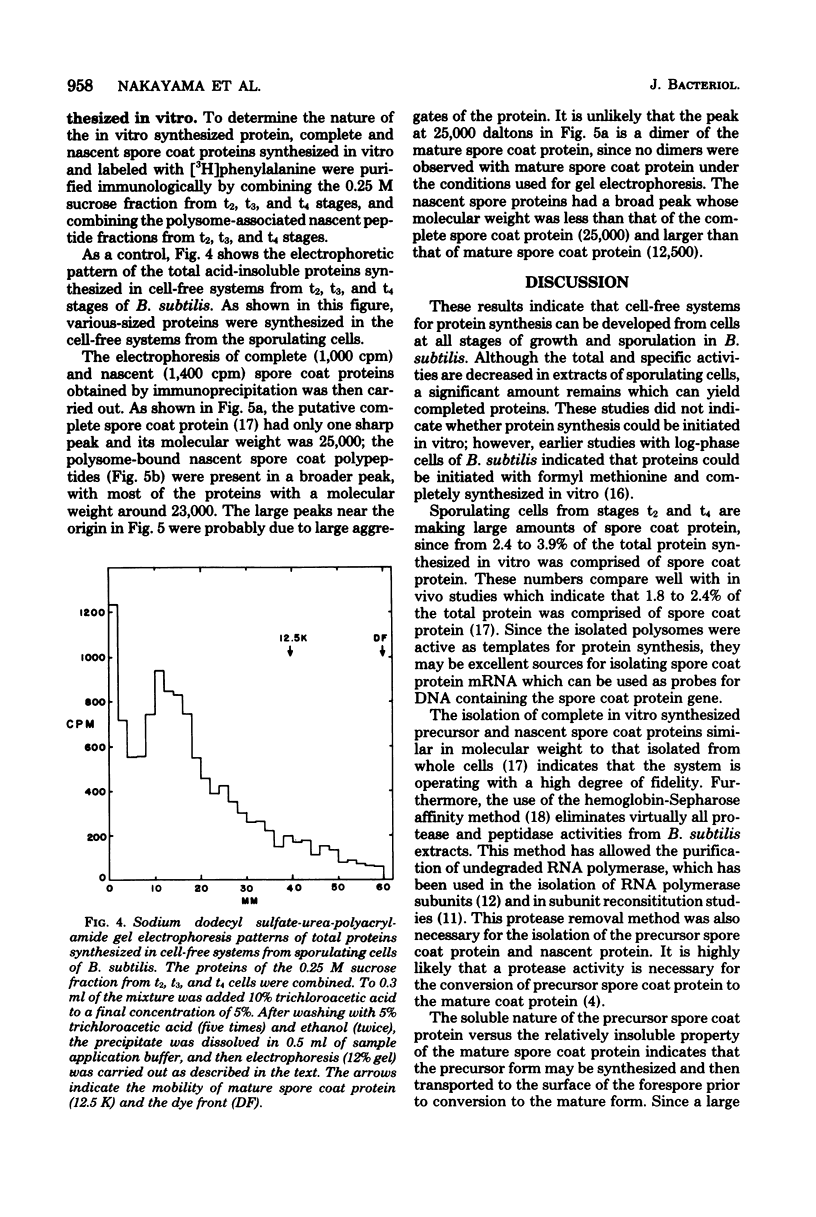
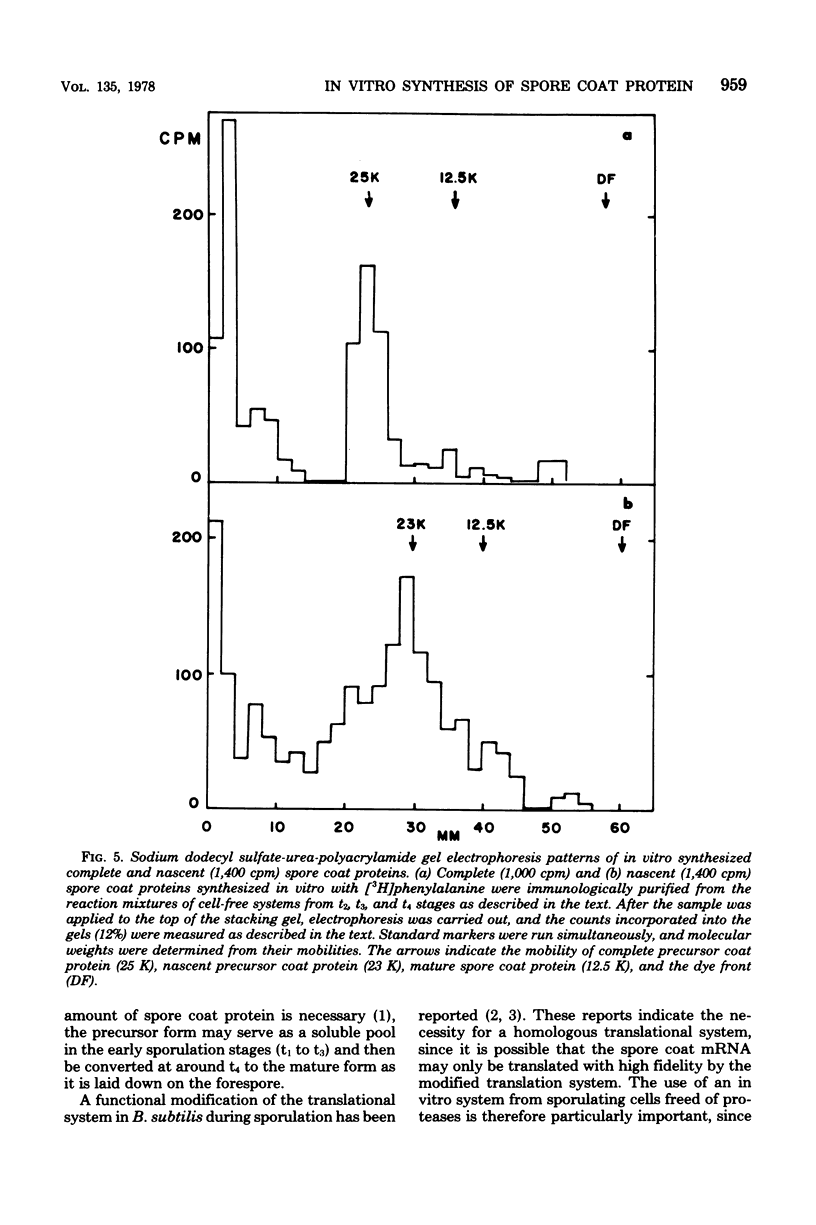
Cell-free systems for protein synthesis were prepared from Bacillus subtilis 168 cells at several stages of sporulation. Immunological methods were used to determine whether spore coat protein could be synthesized in the cell-free systems prepared from sporulating cells. Spore coat protein synthesis first occurred in extracts from stage t2 cells. The proportion of spore coat protein to total proteins synthesized in the cell-free systems was 2.4 and 3.9% at stages t2 and t4, respectively. The sodium dodecyl sulfate-urea-polyacrylamide gel electrophoresis patterns of immunoprecipitates from the cell-free systems showed the complete synthesis of an apparent spore coat protein precursor (molecular weight, 25,000). A polypeptide of this weight was previously identified in studies in vivo (L.E. Munoz, Y. Sadaie, and R.H. Doi, J. Biol. Chem., in press). The synthesis in vitro of polysome-associated nascent spore coat polypeptides with varying molecular weights up to 23,000 was also detected. These results indicate that the spore coat protein may be synthesized as a precursor protein. The removal of proteases in the crude extracts by treatment with hemoglobin-Sepharose affinity techniques may be preventing the conversion of the large 25,000-dalton precursor to the 12,500-dalton mature spore coat protein.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson A. I., Fitz-James P. Structure and morphogenesis of the bacterial spore coat. Bacteriol Rev. 1976 Jun;40(2):360–402. doi: 10.1128/br.40.2.360-402.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambliss G. H., Legault-Demare L. Functional modifications of the translational system in Bacillus subtilis during sporulation. J Bacteriol. 1977 Oct;132(1):13–22. doi: 10.1128/jb.132.1.13-22.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. S., Aronson A. I. Alterations of spore coat processing and protein turnover in a Bacillus cereus mutant with a defective postexponential intracellular protease. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1254–1258. doi: 10.1073/pnas.74.3.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua G. K., Bushuk W. Purification of wheat proteases by affinity chromatography on hemoglobin-Sepharose column. Biochem Biophys Res Commun. 1969 Oct 22;37(3):545–550. doi: 10.1016/0006-291x(69)90950-4. [DOI] [PubMed] [Google Scholar]
- Doi R. H. Role of ribonucleic acid polymerase in gene selection in procaryotes. Bacteriol Rev. 1977 Sep;41(3):568–594. doi: 10.1128/br.41.3.568-594.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAHEY J. L., HORBETT A. P. Human gamma globulin fractionation on anion exchange cellulose columns. J Biol Chem. 1959 Oct;234:2645–2651. [PubMed] [Google Scholar]
- Fukuda R., Doi R. H. Two polypeptides associated with the ribonucleic acid polymerase core of Bacillus subtilis during sporulation. J Bacteriol. 1977 Jan;129(1):422–432. doi: 10.1128/jb.129.1.422-432.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halling S. M., Burtis K. C., Doi R. H. Reconstitution studies show that rifampicin resistance is determined by the largest polypeptide of Bacillus subtilis RNA polymerase. J Biol Chem. 1977 Dec 25;252(24):9024–9031. [PubMed] [Google Scholar]
- Halling S. M., Sanchez-Anzaldo F. J., Fukuda R., Doi R. H., Meares C. F. Zinc is associated with the beta subunit of DNA-dependent RNA polymerase of Bacillus subtilis. Biochemistry. 1977 Jun 28;16(13):2880–2884. doi: 10.1021/bi00632a012. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leighton T. J., Doi R. H. The stability of messenger ribonucleic acid during sporulation in Bacillus subtilis. J Biol Chem. 1971 May 25;246(10):3189–3195. [PubMed] [Google Scholar]
- Linn T., Greenleaf A. L., Losick R. RNA polymerase from sporulating Bacillus subtilis. Purification and properties of a modified form of the enzyme containing two sporulation polypeptides. J Biol Chem. 1975 Dec 25;250(24):9256–9261. [PubMed] [Google Scholar]
- Migita L. K., Doi R. H. The amino-terminal residues of Bacillus subtilis proteins made in vitro. J Biol Chem. 1970 Apr 25;245(8):2005–2010. [PubMed] [Google Scholar]
- Nakayama T., Munoz L., Doi R. H. A procedure to remove protease activities from Bacillus subtilis sporulating cells and their crude extracts. Anal Biochem. 1977 Mar;78(1):165–170. doi: 10.1016/0003-2697(77)90020-3. [DOI] [PubMed] [Google Scholar]
- Nakayama T., Narita K., Ogata K. Biological process of carbohydrate attachment to ovalbumin. J Biochem. 1976 May;79(5):871–881. doi: 10.1093/oxfordjournals.jbchem.a131155. [DOI] [PubMed] [Google Scholar]
- Nakayama T., Ogata K., Narita K. Ovalbumin synthesis in a homologous cell-free system prepared from hen's oviduct. J Biochem. 1976 May;79(5):853–869. doi: 10.1093/oxfordjournals.jbchem.a131154. [DOI] [PubMed] [Google Scholar]
- Schaeffer P. Sporulation and the production of antibiotics, exoenzymes, and exotonins. Bacteriol Rev. 1969 Mar;33(1):48–71. doi: 10.1128/br.33.1.48-71.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G. J., Bruening G. Two proteins from cowpea mosaic virus. Virology. 1971 Dec;46(3):596–612. doi: 10.1016/0042-6822(71)90063-8. [DOI] [PubMed] [Google Scholar]
- Zubay G., Schwartz D., Beckwith J. Mechanism of activation of catabolite-sensitive genes: a positive control system. Proc Natl Acad Sci U S A. 1970 May;66(1):104–110. doi: 10.1073/pnas.66.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]


