Abstract
The Src family of nonreceptor tyrosine kinases are important regulators of a variety of cellular processes, including cytoskeletal organization, cell–cell contact, and cell–matrix adhesion. Activation of Src family kinases also can induce DNA synthesis and cellular proliferation; therefore, tight regulation of their kinase activities is important for the cell to maintain proliferative control. Posttranslational phosphorylation and dephosphorylation are recognized as the principle modifications by which the activities of the Src family of tyrosine kinases are regulated. We have discovered that this family of kinases also is regulated by ubiquitin-mediated proteolysis. Studies aimed at the identification of cellular targets for E6AP, an E3 ubiquitin protein ligase involved in ubquitin-mediated degradation, led us to the identification of members of the Src family kinases as potential substrates for E6AP. We have found that E6AP can bind to several of the Src family tyrosine kinases. Here we show that activated Blk is preferentially degraded by the ubiquitin–proteasome pathway and that its ubiquitination is mediated by E6AP. Identification of members of the Src tyrosine kinase family as substrates of the E6AP ubiquitin-protein ligase implicates a role for the ubiquitin pathway in regulating the activities of individual members of this important family of signaling molecules.
Cellular Src is the prototype of a family of membrane-bound, nonreceptor tyrosine kinases that includes Fyn, Yes, Lyn, Hck, Yrk, Lck, and Blk. These kinases possess quite similar domain structures, including an N-terminal segment that includes the SH4 domain (which is a myristoylation and membrane-localization signal) and 50–70 additional residues that are unique to each family member. This is followed by the SH2 and SH3 regulatory domains, a highly conserved catalytic domain, and a short carboxyl-terminal tail with the regulatory tyrosine residue (1). Src family tyrosine kinases functionally interact and cooperate with several receptor and nonreceptor protein tyrosine kinases to provide a diversity of signals that regulate cellular functions (1, 2). The Src family kinases are themselves tightly regulated, exhibiting little activity in normal cells in the absence of an activating signal. This regulation is mediated by phosphorylation/dephosphorylation of a specific tyrosine residue in the carboxy terminal tail (Tyr 527 in c-Src). Phosphorylation of the regulatory tyrosine residue by cytoplasmic tyrosine kinases such as Csk induces a conformational change that results in a closed (inactive) form of c-Src because of an intramolecular interaction between the phosphorylated tyrosine and the SH2 domain. In this closed conformation, Src is unable to interact with other target proteins through its SH2 and SH3 domains. On the dephosphorylation of Tyr 527, Src adopts an open, active conformation in which the SH2 and SH3 domains are free to interact with other targets. In this open conformation, Tyr 416 in the kinase domain is available for autophosphorylation, further increasing the kinase activity (3). The open and closed conformation model, corresponding to the active and inactive states of the Src family kinases, is compatible with recent structural studies on the human c-Src and Hck kinases (4, 5). To date, however, there has been little work on other cellular processes involved in the regulation of the src family kinases. Although the inactive form of Src family kinases is known to be quite stable, the relative stabilities of the active and inactive forms have not been previously examined.
Ubiquitin-dependent proteolysis has been implicated in a variety of cellular processes, including DNA repair, cell cycle control, antigen presentation, chromosomal organization, intracellular translocation of proteins, and apoptosis (6). The covalent conjugation of multiple ubiquitin molecules to lysine residues of a target protein serves to signal its recognition and rapid degradation by the 26S proteasome (6, 7). Ubiquitination of protein substrates is a multistep process that involves the concerted action of at least three classes of enzymes: ubiquitin activating enzyme E1, ubiquitin conjugating enzymes (E2s), and ubiquitin protein ligases (E3s) (6). The biochemical mechanisms of ubiquitin transfer within the enzymatic components of the pathway and its subsequent conjugation to target proteins are now understood in considerable detail. E1 first activates ubiquitin in an ATP-dependent reaction through the formation of ubiquitin adenylate, followed by a thiolester bond between the carboxyl terminus of ubiquitin and thiol group of a specific cysteine residue in E1. Ubiquitin then is transferred to a specific cysteine residue in one of several E2s (8). E2 enzymes, in turn, either transfer the ubiquitin directly to a substrate or to E3 ubiquitin protein ligases, which catalyze the formation of an isopeptide bond between the carboxyl terminus of ubiquitin and the ɛ-amino group of specific lysine residues on a target protein (6, 9, 10). A substrate may be multiply ubiquitinated by sequential linkage of additional ubiquitin molecules to each other through specific lysine residues (K48 or K63). Multiubiquitination of a protein leads to its recognition and consequent degradation by the 26S proteasome (6, 7).
The E3 ubiquitin protein ligases are the key components that provide specificity to the ubiquitin system by direct interaction with the substrates. E6AP was the first E3 mammalian ubiquitin protein ligase to be recognized and characterized. E6AP was initially identified as a 100-kDa cellular protein that, in conjunction with the E6 oncoprotein of human papilloma virus type 16 (HPV), constitutes the E3 activity in the ubiquitination of p53 (9, 11–13). E6AP also was found to promote the ubiquitination of cellular proteins in the absence of E6, indicating that E6AP could function as an E3 enzyme independent of E6 (9). E6AP is the prototype of a family of structurally and functionally related proteins from various organisms defined by a region of ≈350 amino acids in the carboxyl terminus, which is designated the HECT (homologous to the E6AP carboxyl terminus) domain (14). These E3 enzymes are capable of forming a thiolester bond with ubiquitin and can transfer the ubiquitin moiety to specific protein substrates (10, 14). As noted above, the specificity of substrate recognition by the ubiquitin system is achieved by E3 enzymes through direct interaction with specific substrates. In the case of Hect proteins, for instance, their divergent amino terminal sequences may provide the necessary diversity required for substrate recognition whereas their conserved carboxyl terminus (Hect domain) can interact with specific E2 enzymes and can catalyze the ubiquitination of bound substrates (15–17).
To date, only a small number of proteins have been identified as substrates of Hect E3 enzymes. The general amino acid permease Gap1 and uracil permease Fur4 have been reported to be ubiquitinated by RSP5 (NPI1), a Hect protein of Saccharomyces cerevisiae (18, 19). The large subunit of RNA polymerase II (Rpb1) was recently identified also as an RSP5 substrate (16). In addition, the Schizosaccharomyces pombe homologue of RSP5, Pub1, has been shown to target the CDC25 phosphatase for ubiquitin-dependent degradation (20). In the case of E6AP, p53 recognition and ubiquitination depends on the presence of the oncogenic HPV E6 protein (21). In the absence of E6, the ubiquitination of p53 is not governed by E6AP but, rather, depends on Mdm2 (22, 23). Interestingly, E6AP was recently identified as the gene affected in Angelman syndrome, a genetic neurological disorder. A majority of the mutations in E6AP is predicted to abolish the catalytic activity of E6AP, raising the possibility that deregulation of E6AP substrates may contribute to the pathogenesis of Angelman syndrome (24, 25). We have therefore been interested in identifying the cellular substrates of E6AP. Using a catalytically inactive form of E6AP in the yeast two-hybrid system (17), we have identified the human homologues of yeast Rad23 (HHR23A and HHR23B) as interacting proteins and as substrates both in vitro and in vivo (26).
In this study, we present our identification of the Src family kinases, Lck and Blk, as E6AP interacting proteins. To address the physiologic significance of these interactions, we have studied the regulation of Blk. We have found that activated Blk is preferentially degraded by the ubiquitin–proteasome pathway and that its ubiquitination is mediated by E6AP. The identification of Blk and several other members of the Src tyrosine kinase family as substrates of the E6AP ubiquitin–protein ligase suggests that ubiquitination may be a previously unrecognized mechanism for regulating the activities of individual members of this kinase family. Although we have not yet determined the relevance of the HHR23 proteins or the src family kinases to the pathogenesis of Angelman syndrome, the identification of the normal cellular substrates of E6AP should provide insight into the normal cellular functions of E6AP that may be dysfunctional in this neurologic disorder.
MATERIALS AND METHODS
Plasmids and Antibodies.
Mammalian expression vectors containing mouse Blk, BYF [Blk (Y495F)], constitutively active form of kinase and BKR [Blk (K263R)] disrupted at the ATP binding site of the kinase were provided by Steve Desiderio (Johns Hopkins University). Blk and Lck cloned into pGEM-1 were kindly gifted by Joseph Bolen (Hoechst Marion Roussel) (27). Bacterial expression vector containing glutathione S-transferase (GST)–E6AP, GST-E6AP (C833A), mammalian expression vectors containing HA-tagged E6AP (wild-type or C833A), and anti-E6AP antibodies were described previously (21). Rabbit anti-mouse Blk antibody was obtained from Santa Cruz Biotechnology. Monoclonal anti-ubiquitin antibody was purchased from Zymed.
Cell Culture, Transient Transfection, and Kinase Activation.
Cos-7 cells (American Type Culture Collection) and LS102.9 cells (murine B cell line, American Type Culture Collection) were grown in DMEM containing 10% fetal bovine serum in 5% CO2 at 37°C. Transient transfections were performed by the standard calcium phosphate methods, and kinase activation was done 40 hr after transfection. To active Blk kinase activity, cells were treated with pervanadate or anti-IgM antibody. Pervanadate was freshly prepared by mixing 10 mM of sodium orthovanadate (Sigma) solution with 10 mM H2O2 for 20 min at room temperature, followed by the addition of catalase (Sigma) at the final concentration of 200 μg/ml to remove residual H2O2. Pervanadate generated by this procedure is reported to be stable for 2 hr without further addition of H2O2 (28–30). Receptor ligation was performed as described (31, 32) with 5 × 107 LS102.9 cells at 37°C in 1 ml of serum-free DMEM, using 30 μg of rabbit anti-mouse IgM (Zymed). To inhibit ubiquitin-dependent proteolysis, cells were treated with MG132 (Peptide Institute, Osaka) at a final concentration of 25 or 40 μM, as indicated. Cells were lysed in Nonidet P-40 lysis buffer (50 mM Tris⋅HCl, at pH 8.0/150 mM NaCl/1% Nonidet P-40). Insoluble debris was removed by centrifugation, and the detergent-soluble cell lysates were used as total cell lysates in the subsequent procedure.
In Vitro Binding Assays.
Blk and Lck were labeled with [35S] cysteine and methionine by in vitro transcription and translation in rabbit reticulocyte lysate (Promega) and were mixed with GST-E6AP (wild-type or C833A form), or GST alone in lysis buffer (0.1 M NaCl/0.1 M Tris⋅HCl, pH 7.4/1% Nonidet P-40). The mixtures were rotated at 4°C for 4 hr. The beads were collected by centrifugation and were washed three times with lysis buffer, and bound Blk or Lck were analyzed by 9% SDS/PAGE and autoradiography.
Immunopreciptation and Western Blot Analysis.
Immunoprecipitation was carried out in RIPA buffer (50 mM Tris⋅HCl, pH 8.0/150 mM NaCl/1% Nonidet P-40/0.5% deoxycholate/0.1% SDS). Antibodies were incubated with total cell lysates at 4°C overnight. Protein A-agarose (GIBCO/BRL) equilibrated in the same buffer then was added to the mixture, and the incubation was continued for another 1.5 hr. Whole cell lysates or immunoprecipitates were separated on 7.5% SDS/PAGE gels and were transferred to poly(vinylidene difluoride) membrane (NEN). Western blots were performed by standard procedures using enhanced chemiluminescence (NEN).
In Vitro Kinase Assay.
The immune complex precipitated with anti-Blk antibodies/protein A-agarose was washed three times with RIPA buffer and twice with the kinase buffer (50 mM Tris≈HCl, pH 7.4/10 mM MgCl2/5 mM MnCl2/1 mM DTT) and then was resuspended in 50 μl of kinase buffer containing 1 μCi of γ-32P-labeled ATP (NEN). The reaction mixture was incubated at 30°C for 15 min with continuous mixing. The beads then were washed four times with lysis buffer, and the reaction was stopped by addition of SDS sample buffer. The samples were boiled for 5 min and were subjected to 7.5% polyacrylamide-SDS gel, followed by autoradiography.
RESULTS
E6AP Interacts with Members of the Src Family of Tyrosine Kinases.
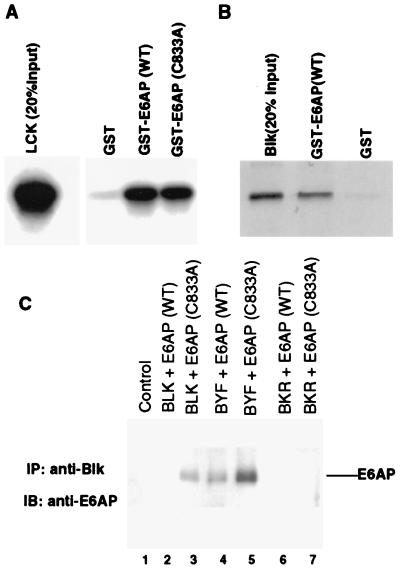
Using the yeast two-hybrid system with a catalytically inactive form of E6AP as bait to screen a human T cell library, we isolated Lck as an E6AP-interacting protein (data not shown). To confirm this interaction, we performed in vitro binding assays with Lck and bacterially expressed GST-E6AP. 35S-labeled in vitro synthesized Lck was mixed with bacterially produced GST fusion proteins of wild-type or dominant negative (C833A) forms of E6AP (21). As shown in Fig. 1A, ≈10% of input Lck bound to GST-E6AP. We performed similar binding assays with Blk, a closely related Src family kinase expressed in B cells, to assess whether the interaction with E6AP was limited to Lck or a general property shared with other members of the Src family kinases. As shown in Fig. 1B, ≈20% of input Blk bound to E6AP, indicating that additional members of the Src family also can interact with E6AP.
Figure 1.
In vitro and in vivo binding of Lck and Blk to E6AP. (A) In vitro binding of Lck to E6AP. Lck was translated in vitro in the presence of [35S]cysteine and methionine and was mixed with bacterially produced GST, GST-E6AP, or GST-E6AP (C833A) immobilized on glutathione-Sepharose. The complexes were washed to remove noninteracting proteins and were analyzed by SDS/PAGE and autoradiography. (B) In vitro binding of Blk to E6AP. 35S-labeled, in vitro translated Blk was mixed with the indicated GST or GST-E6AP proteins. After immobilization on glutathione-Sepharose, the complexes were washed and analyzed by SDS/PAGE and autoradiography. (C) In vivo interaction of E6AP and Blk by co-immunoprecipitation assay. Cos-7 cells were cotransfected by the calcium phosphate method with HA-tagged E6AP (wild-type) or E6AP (C833A) and Blk, BYF [Blk (Y495F), constitutively active form] or BKR [Blk (K263R), kinase-defective form] as indicated. After 40 hr, total cell lysates were immunoprecipitated with anti-Blk antibody followed by immunoblotting with a rabbit polyclonal anti-E6AP antibody.
E6AP Preferentially Binds Activated Forms of Blk.
We used several previously characterized kinase mutants of Blk to extend these in vitro observations and to determine whether the activation state of the Src family kinases influenced their abilities to bind E6AP. The ability of wild-type, kinase-defective, and constitutively active forms of Blk to bind E6AP was assessed by cotransfection of E6AP and Blk expression plasmids into Cos-7 cells. The mutant Blk constructs used were BKR (K263R), a kinase-defective mutant due to the lysine to arginine substitution in the ATP-binding domain, and BYF (Y495F), a constitutively active kinase caused by the substitution of the regulatory tyrosine. After immunoprecipitation with anti-Blk antibodies, immunoblots were probed with anti-E6AP antibodies to detect associated E6AP (Fig. 1C). These experiments used wild-type (wt) E6AP as well as a dominant negative mutant of E6AP (C833A) because of the alanine substitution of the catalytic cysteine (21). Immunoblot analysis of the transfected cell lysates demonstrated approximately equivalent levels of the BYF, BKR, and wt Blk proteins and of the wt and C833A forms of E6AP (data not shown). BYF bound most efficiently to E6AP (C833A) and showed significant binding to wt E6AP. A definite interaction also was observed between wt Blk and the dominant negative form of E6AP, with only slight binding to wt E6AP. Of interest, no binding was observed between the kinase-defective BKR and either form of E6AP. These results confirmed our in vitro data and suggested that E6AP associates preferentially with the active form of Blk. The lower levels of binding observed in this experiment with wt E6AP compared those seen with dominant negative E6AP (C833A) likely reflects the E3 function of E6AP in mediating the ubiquitination and proteolysis of the bound fraction of Blk and BYF as substrates (see below).
Activated Blk Is Less Stable.
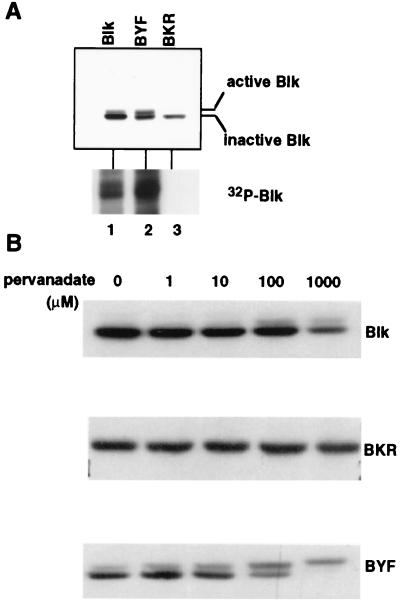
We next examined the relative stabilities of the kinase active and inactive forms of Blk. Forty hours after transfection of Cos-7 cells with plasmids expressing Blk, BYF or BKR, whole cell lysates were prepared and immunoblots performed by using anti-Blk antibodies. Two different forms of Blk were readily distinguished by PAGE migration, presumably because of phosphorylation events associated with kinase activation. As shown in lane 1 of Fig. 2A, the majority of Blk was detected as a 55-kDa band with only a small proportion of the slower migrating species. The ratio of the upper and lower bands in BYF appeared approximately equal. In contrast, only the lower 55-kDa band was observed with the kinase inactive BKR. These migration patterns and the levels of the upper phosphorylated bands correlated with the kinase activities as determined by in vitro kinase assays (Fig. 2A Lower). Approximately 5-fold higher kinase activity was associated with BYF compared with that of Blk, and no kinase activity was found associated with BKR. Taken together, these results suggest that the slower migrating bands represent the active, autophosphorylated forms of the kinase.
Figure 2.
Expression levels of Blk constructs in Cos-7 cells and their associated kinase activities. (A) Active and inactive forms of Blk have distinct mobilities. Extracts from Cos-7 cells transfected with Blk, BYF, and BKR were analyzed by immunoblot and immune-complex kinase assay. (B) Degradation susceptibilities of Blk constructs after pervanadate treatment were correlated with their kinase activities. Cos-7 cells were transfected with Blk constructs as indicated. After 2 hr of treatment with increasing concentration of pervanadate, equivalent amounts of protein from total cell lysates were immunoblotted with anti-Blk antibody.
The preferential binding of E6AP to the activated forms of Blk led us to examine the possibility that active Blk might be degraded through the ubiquitin–proteasome pathway. Plasmids expressing BKR, BYF, or Blk were transfected into Cos-7 cells that then were treated with pervanadate to activate the tyrosine kinase activity. Protein levels of the kinase active and inactive forms of Blk then were analyzed by immunoblot assay of cellular extracts by using anti-Blk antibodies. As shown in Fig. 2B, BKR was completely resistant to pervanadate activation in that no slower migrating form could be detected at any of the concentrations of pervanadate tested. In contrast, with increasing concentrations of pervanadate, conversion of the lower forms of Blk and of BYF to the upper, autophosphorylated forms was observed. Furthermore, the overall levels of Blk and BYF decreased on pervanadate treatment in a dose-dependent manner whereas the levels of the kinase inactive BKR protein remained unchanged. These findings further support the model that the activation of the Blk kinase promotes its proteolysis.
Activated Blk Is a Substrate for Ubiquitin-Mediated Proteolysis.
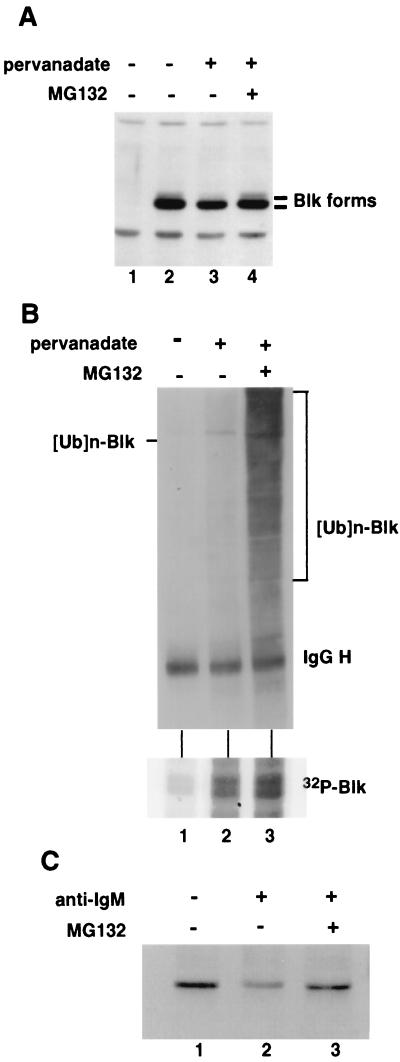
We next determined whether the degradation that we observed for activated Blk occurred through the ubiquitin–proteasome pathway. We examined the effect of the proteasome inhibitor, MG132, on pervanadate-mediated activation and degradation of Blk. Cos-7 cells transfected with Blk were treated with pervanadate (100 μM) with or without MG132. In agreement with the results shown in Fig. 2, treatment with pervanadate resulted in a preferential decrease in the level of the kinase active forms of Blk (Fig. 3A, lanes 2 and 3). Treatment of the cells with MG132 resulted in the stabilization of the slower migrating species of Blk in the cells treated with pervanadate (Fig. 3A, lane 4), indicating that the activated form of Blk is degraded through the ubiquitin–proteasome pathway.
Figure 3.
Stabilization of the activated form of Blk by the proteasome inhibitor (MG132) and detection of multiubiquitinated forms of Blk. (A) Western blot of Cos-7 cells transfected with Blk with or without pervanadate (100 μM) and MG132 (25 μM). Total cell lysates were probed with anti-Blk antibody and were subjected to immunoprecipitation with the same antibody and immune-complex kinase assay. (B) Blk becomes ubiquitinated on pervanadate treatment. Cos-7 cells transfected with Blk were treated with or without pervanadate (100 μM) and MG132 (25 μM) for 2 hr. Total cell lysates (1.5 mg) were immunoprecipitated with anti-Blk antibody, and ubiquitinated Blk was detected by Western blot analysis with antiubiquitin antibody. (C) Degradation of endogenous Blk by kinase activation and stabilization with MG132 treatment. The LS102.9 cells (2 × 107) were incubated in the absence or presence of antibody against murine IgM (30 μg/ml) with or without MG132 (40 μM) as indicated. The lysates were immunoprecipitated with anti-Blk antibody conjugated with protein A agarose beads, then were subjected to immunoblotting for Blk.
To substantiate further the involvement of ubiquitin-mediated proteolysis in the regulation of Blk, we looked for ubiquitin-Blk conjugate. Immunoprecipitations of Blk from transfected Cos-7 cell extracts were examined by Western blot analysis by using antiubiquitin antibodies. As shown in Fig. 3B, ubiquitination of Blk was observed in cells treated with pervanadate, correlating with the activation of its kinase activity (Fig. 3B Lower). Pervanadate treatment alone resulted in the appearance of faint higher molecular bands corresponding to multiubiquitinated forms of Blk. The combination of pervanadate and MG132 greatly enhanced the intensity of these bands, resulting in a smear of higher molecular weight forms characteristic of multiubiquitination.
To extend these findings to endogenous Blk, we examined the expression of Blk in several B cell lines. Because the levels of expression of endogenous Blk were found to be relatively low (data not shown) and because the migration of endogenous Blk (55 kDa) is obscured by the Ig heavy chains, we used Blk antibodies conjugated with agarose beads for immunoprecipitations followed by immunoblot analysis. In B-lymphocytes, Blk is associated with the cytoplasmic tail of IgM and can be activated with anti-IgM antibodies through complexing cell surface IgM molecules (31, 32). Treatment of the murine B cell line LS102.9 with anti-IgM antibodies resulted in a significant and reproducible decrease in Blk protein levels (Fig. 3C, compare lanes 1 and 2). Furthermore, the degradation of Blk after its activation by anti-IgM antibodies was blocked by treatment of the cells with MG132 (Fig. 3C, lane 3). Similar results were observed with pervanadate treatment in LS102.9 cells (data not shown). In these experiments, no definitive upper band corresponding to the activated Blk was observed, presumably because of the low amount of endogenous Blk in the cells and modest levels of kinase activation achieved (data not shown). Nonetheless, these experiments demonstrate that the physiologic activation of endogenous Blk results in its proteasome-mediated degradation.
E6AP Can Mediate the Proteolysis of Activated Blk.
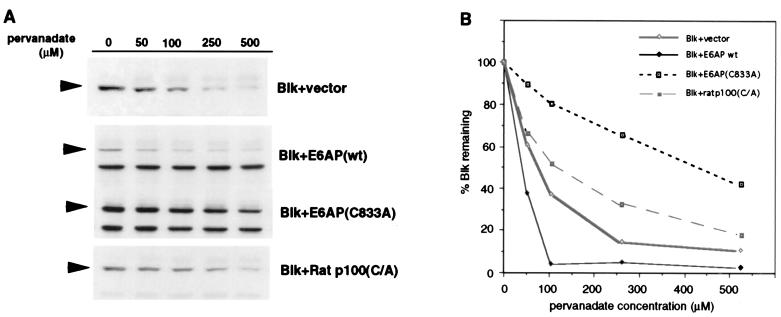
To confirm an in vivo role for E6AP in the degradation of the active form of the Blk kinase, Blk protein levels were examined in pervanadate treated Cos-7 cells cotransfected with either the wt or the dominant negative, catalytically inactive C833A form of E6AP. Cotransfection of Blk with wt E6AP enhanced the degradation of pervanadate-activated Blk (compared with Blk in cells cotransfected with the vector control) (Fig. 4). In addition, the C833A dominant negative form of E6AP inhibited the degradation of activated Blk, providing evidence that E6AP mediates the proteolysis in vivo of activated Blk. As a specificity control in this experiment, we analyzed the effect of Ratp100, another Hect protein distinct from E6AP, which had been similarly mutated by alanine substitution of the cysteine corresponding to Cys833 of E6AP (17). The degradation of Blk was only slightly inhibited by cotransfection of Ratp100 C/A mutant compared with the vector control (Fig. 4). The small effect observed with the Ratp100 C/A mutant is possibly caused by nonspecific squelching of upstream components of the ubiquitin pathway, such as the E1 ubiquitin activating enzymes or specific E2 ubiquitin conjugating enzymes. Taken together, these data provide evidence for a direct involvement of E6AP in the degradation of activated Blk.
Figure 4.
Effect of wild-type and dominant negative E6AP on the degradation of activated Blk. Blk was transfected into Cos-7 cells with wild-type or C833A forms of E6AP, the catalytically inactive form (C/A mutant) of the rat p100 E3, or empty vector. Forty hours after transfection, cells were treated with increasing concentrations of pervanadate for 2 hr. Total cell lysates were subjected to immunoblot analysis for Blk (A). Specific signals (arrow heads) were quantitated by densitometry, and the percent Blk remaining at each concentration of pervanadate was compared with the nontreated levels (B).
DISCUSSION
In this study, we have demonstrated that the ubiquitin–proteasome pathway selectively degrades the activated form of Blk. This process involves E6AP, which preferentially binds to the active form of Blk and promotes its ubiquitination and proteolysis. Blk is stable without activation of its kinase activity, and a kinase-defective mutant of Blk remains stable even after pervanadate treatment. Furthermore, multiubiquitinated forms of Blk can be observed only after activation of the kinase. These results indicate that activation of Blk kinase signals its recognition by the ubiquitin machinery through binding to E6AP, which in turn leads to its ubiquitination and proteolysis.
The Src family tyrosine kinases have important roles in a number of signal transduction pathways, and their activity must be tightly regulated. Phosphorylation and dephosphorylation of the regulatory tyrosine residue is the principal mechanism that has been recognized in the regulation of the Src family kinases. Dephosphorylation causes a switch from a closed to an open conformation, thereby allowing the catalytic domain to bind its kinase substrates. The kinase can be converted back to the inactive closed conformation by phosphorylation of the regulatory tyrosine by Csk (33). Our findings, however, provide evidence for an additional mechanism to turn off and limit the activity of the Src family kinases through the targeted ubiquitin-dependent degradation of their activated forms. The irreversible nature of proteolysis makes it an ideal mechanism to limit the activity of signaling molecules such as the Src family kinases.
Although this study has mainly examined Blk, our preliminary data suggest ubiquitin-mediated proteolysis also may be applicable to at least some of the other Src family tyrosine kinases. In fact, the first evidence that this family of kinases might be so regulated came from our observation that E6AP interacts with Lck. We also have preliminary data that E6AP also can bind Src in GST pull down experiments (K. Harris, E. Cooper, and P.M.H., unpublished work). We are currently investigating the possibility that other members of the Src family might be regulated in a similar manner. This possibility is consistent with the observations that the kinase activity of Src family members (Src, Fyn, and Yes) is extremely high and that the steady state levels of these proteins are quite low in Csk−/− cells (34, 35). Our preliminary experiments indicate that the lower steady state levels of the src family kinases in Csk−/− cells may result from decreased protein stability (data not shown).
Further analyses will be necessary to elucidate the physiological importance of the ubiquitin–proteasome pathway on the regulation of the other Src family tyrosine kinases. It also will be important to examine what effect, if any, the papillomavirus E6 proteins may have on the stability of the Src family kinases. E6 promotes the degradation of p53 by directing E6AP to p53; in the absence of E6, p53 is not a substrate of E6AP (21, 36). It is possible that, in targeting E6AP to p53, E6 redirects E6AP away from its normal cellular substrates, increasing their stability. One can envisage that the stabilization of the activated forms of specific members of the Src family of kinases could contribute to the HPV transformed phenotype.
Acknowledgments
We thank S. Desiderio and J. Bolen for the plasmids expressing the individual src family kinases. We are grateful to J. Bolen, J. Benson, L. Ronco, and K. Harris for a critical review of this manuscript. This work was supported by a grant from the National Institutes of Health (RO1-CA64888) to P.M.H. SK was supported by a postdoctoral fellowship from the American Cancer Society (PF-4309).
ABBREVIATIONS
- wt
wild-type
- GST
glutathione S-transferase
References
- 1.Brown M T, Cooper J A. Biochim Biophys Acta. 1996;1287:121–149. doi: 10.1016/0304-419x(96)00003-0. [DOI] [PubMed] [Google Scholar]
- 2.Parsons J T, Parsons S J. Curr Opin Cell Biol. 1997;9:187–192. doi: 10.1016/s0955-0674(97)80062-2. [DOI] [PubMed] [Google Scholar]
- 3.Cooper J A, Howell B. Cell. 1993;73:1051–1054. doi: 10.1016/0092-8674(93)90634-3. [DOI] [PubMed] [Google Scholar]
- 4.Xu W, Harrison S C, Eck M J. Nature (London) 1997;385:595–602. doi: 10.1038/385595a0. [DOI] [PubMed] [Google Scholar]
- 5.Sicheri F, Moarefi I, Kuriyan J. Nature (London) 1997;385:602–609. doi: 10.1038/385602a0. [DOI] [PubMed] [Google Scholar]
- 6.Hochstrasser M. Annu Rev Genet. 1996;30:405–439. doi: 10.1146/annurev.genet.30.1.405. [DOI] [PubMed] [Google Scholar]
- 7.Pickart C M. FASEB J. 1997;11:1055–1066. doi: 10.1096/fasebj.11.13.9367341. [DOI] [PubMed] [Google Scholar]
- 8.Jentsch S. Annu Rev Genet. 1992;26:179–207. doi: 10.1146/annurev.ge.26.120192.001143. [DOI] [PubMed] [Google Scholar]
- 9.Scheffner M, Huibregtse J M, Vierstra R D, Howley P M. Cell. 1993;75:495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- 10.Scheffner M, Nuber U, Huibregtse J. Nature (London) 1995;373:81–83. doi: 10.1038/373081a0. [DOI] [PubMed] [Google Scholar]
- 11.Huibregtse J M, Scheffner M, Howley P M. EMBO J. 1991;10:4129–4135. doi: 10.1002/j.1460-2075.1991.tb04990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huibregtse J M, Scheffner M, Howley P M. Mol Cell Biol. 1993;13:775–784. doi: 10.1128/mcb.13.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheffner M, Werness B A, Huibregtse J M, Levine A J, Howley P M. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 14.Huibregtse J, Scheffner M, Beaudenon S, Howley P. Proc Natl Acad Sci USA. 1995;92:2563–2567. doi: 10.1073/pnas.92.7.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huibregtse J M, Scheffner M, Howley P M. Mol Cell Biol. 1993;13:4918–4927. doi: 10.1128/mcb.13.8.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huibregtse J M, Yang J C, Beaudenon S L. Proc Nat Acad Sci USA. 1997;8:3656–3661. doi: 10.1073/pnas.94.8.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar S, Kao W H, Howley P M. J Biol Chem. 1997;272:13548–13554. doi: 10.1074/jbc.272.21.13548. [DOI] [PubMed] [Google Scholar]
- 18.Galan J M, Moreau V, Andre B, Volland C, Haguenauer-Tsapis R. J Biol Chem. 1996;271:10946–10952. doi: 10.1074/jbc.271.18.10946. [DOI] [PubMed] [Google Scholar]
- 19.Hein C, Springael J, Volland C, Haguenauer-Tsapis R, Andre B. Mol Microbiol. 1995;18:77–87. doi: 10.1111/j.1365-2958.1995.mmi_18010077.x. [DOI] [PubMed] [Google Scholar]
- 20.Nefsky B, Beach D. EMBO J. 1996;15:1301–1312. [PMC free article] [PubMed] [Google Scholar]
- 21.Talis A L, Huibregtse J M, Howley P M. J Biol Chem. 1998;273:6439–6445. doi: 10.1074/jbc.273.11.6439. [DOI] [PubMed] [Google Scholar]
- 22.Kubbutat M H G, Jones S N, Vousden K H. Nature (London) 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 23.Haupt Y, Maya R, Kazaz A, Oren M. Nature (London) 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 24.Kishino T, Lalande M, Wagstaff J. Nat Genet. 1997;15:70–73. doi: 10.1038/ng0197-70. [DOI] [PubMed] [Google Scholar]
- 25.Matsuura T, Sutcliffe J S, Fang P, Galjaard R J, Jiang Y H, Benton C S, Rommens J M, Beaudet A L. Nat Genet. 1997;15:74–77. doi: 10.1038/ng0197-74. [DOI] [PubMed] [Google Scholar]
- 26.Kumar S, Talis A L, Howley P M. J Biol Chem. 1999;274:18785–18792. doi: 10.1074/jbc.274.26.18785. [DOI] [PubMed] [Google Scholar]
- 27.Mahajan S, Fargnoli J, Burkhardt A L, Kut S A, Saouaf S J, Bolen J B. Mol Cell Biol. 1995;15:5304–5311. doi: 10.1128/mcb.15.10.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kadota S, Fantus I G, Deragon G, Guyda H J, Hersh B, Posner B I. Biochem Biophys Res Commun. 1987;147:259–266. doi: 10.1016/s0006-291x(87)80115-8. [DOI] [PubMed] [Google Scholar]
- 29.Fantus I G, Kadota S, Deragon G, Foster B, Posner B I. Biochemistry. 1987;28:8864–8871. doi: 10.1021/bi00448a027. [DOI] [PubMed] [Google Scholar]
- 30.Secrist J P, Burns L A, Karnitz L, Koretzky G A, Abraham R T. J Biol Chem. 1993;268:5886–5893. [PubMed] [Google Scholar]
- 31.Burkhardt A, Brunswick M, Bolen J, Mond J. Proc Natl Acad Sci USA. 1991;88:7410–7414. doi: 10.1073/pnas.88.16.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saouaf S J, Mahajan S, Rowley R B, Kut S, Fargnoli J, Burkhardt A L, Tsukada S. Proc Natl Acad Sci USA. 1994;91:9524–9528. doi: 10.1073/pnas.91.20.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okada M, Nada S, Yamanashi Y, Yamamoto T, Nakagawa H. J Biol Chem. 1991;266:24249–24252. [PubMed] [Google Scholar]
- 34.Nada S, Yagi T, Takeda H, Tokunaga T, Nakagawa H, Ikawa Y, Okada M, Aizawa S. Cell. 1993;73:1125–1135. doi: 10.1016/0092-8674(93)90642-4. [DOI] [PubMed] [Google Scholar]
- 35.Imamoto A, Soriano P. Cell. 1993;73:1117–1124. doi: 10.1016/0092-8674(93)90641-3. [DOI] [PubMed] [Google Scholar]
- 36.Beer-Romano P, Glass S, Rolfe M. Oncogene. 1997;14:595–602. doi: 10.1038/sj.onc.1200872. [DOI] [PubMed] [Google Scholar]