Abstract
We have previously demonstrated the full-length gonococcal transferrin binding proteins (TbpA and TbpB) to be promising antigens in the development of a protective vaccine against Neisseria gonorrhoeae. In the current study we employed a genetic chimera approach fusing domains from TbpA and TbpB to the A2 domain of cholera toxin, which naturally binds in a non-covalent fashion to the B subunit of cholera toxin during assembly. For one construct, the N-terminal half of TbpB (NB) was fused to the A2 subunit of cholera toxin. In a second construct, the loop 2 region (L2) of TbpA was genetically fused between the NB domain and the A2 domain, generating a double chimera. Both chimeras were immunogenic and induced serum bactericidal and vaginal growth-inhibiting antibodies. This study highlights the potential of using protective epitopes instead of full-length proteins in the development of an efficacious gonococcal vaccine.
Keywords: TonB-dependent transporter, Neisseria gonorrhoeae, iron, cholera toxin, vaccine
1. Introduction
The sexually transmitted disease gonorrhea is a global health concern, with increasing infection rates in many parts of the world [1]. The WHO estimates that there are more than 62 million new cases of gonorrhea per year [2]. In 2004, the CDC reported approximately 330,000 new cases of gonorrhea in the U.S. [3]. Both of the above approximations are suggested to be underestimated by almost half due to inadequate reporting measures, as well as the prevalence of asymptomatic infection [1]. In men, hallmark symptoms include urethral mucopurulent discharge and dysuria. Often women suffer asymptomatic infections, with no overt signs or symptoms of the disease [4]. Symptomatic infections in women can include purulent vaginal discharge, dysuria, intermenstrual bleeding, and menorrhagia [5]. Complications in women are common and can include ascension into the upper genital tract resulting in pelvic inflammatory disease (PID), which can lead to fallopian tube scarring. This can cause sterility or decreased fertility, and/or ectopic pregnancy.
Gonorrhea can be effectively treated with antibiotics. However, as with most bacteria under antibiotic pressure, gonococcal antibiotic resistance has emerged [6]. This has resulted in the use of newer and more expensive antibiotics for treating this disease. In addition to multiple-drug resistant organisms, a disturbing finding concerning HIV and gonococcal co-infection has been reported. Studies have demonstrated an increase in HIV titers in the mucosal secretions of both males and females during gonococcal/HIV co-infection [7, 8]. Co-infected individuals thus increase the probability of infecting other sexual partners with HIV. For these reasons, the search for an effective gonococcal vaccine has become more imperative.
There have been a number of attempts to develop vaccines to prevent gonococcal disease. Vaccines tested in humans using partially-lysed gonococci, pilin, and porin all failed, likely due to antigenic variation of these or surrounding surface structures [9–11]. These failed attempts have prompted researchers to look for surface antigens that are conserved in sequence from strain to strain, and not subject to high frequency variation. The transferrin binding proteins fit these criteria [12, 13]. Vaccine studies using meningococcal Tbps have demonstrated elicitation of antibodies that are cross-reactive against heterologous strains, are bactericidal, and can block transferrin utilization [14–17]. Furthermore, in a meningococcal mouse model, mice immunized with TbpA or TbpA and TbpB were completely protected from lethal challenge [16]. These studies suggest that the neisserial transferrin binding proteins could serve as protective antigens to prevent neisserial diseases. A mutant lacking the transferrin binding proteins was unable to colonize the urethra or cause symptoms of urethritis in a human male challenge model of gonococcal infection [18]. These data, in conjunction with the vaccine studies in meningococcal models, represent strong evidence that the transferrin binding proteins could be an ideal target in the development of a protective gonococcal vaccine.
We demonstrated previously that intranasal immunization with the gonococcal transferrin-binding proteins chemically conjugated to the cholera toxin B subunit (Ctb) induced systemic and vaginal antibodies against both TbpA and TbpB [19]. Furthermore, we demonstrated bactericidal activity of immune sera from mice immunized with these Tbp-Ctb chemical conjugates [19]. One of the drawbacks to using the chemical conjugation of two proteins however is the heterologous nature of the vaccine preparation due to differential cross-linking of two proteins. The adjuvanticity of Ctb is dependent on its ability to bind GM1 ganglioside [20]. One potential pitfall of a Ctb chemically conjugated vaccine is that a portion of the vaccine preparation may not be able to bind to its receptor due to steric hindrance caused by a large co-conjugated protein such as a transferrin binding protein. Furthermore, incorporation of full-length proteins in a vaccine antigen preparation has the potential to generate a diverse antibody response, comprising both protective and diversional antibodies. Immunogenic epitopes tend to vary in sequence between strains, due to immunologic pressure, and to result in non-functional antibody deposition on the cell surface. Thus, identification of the protective epitopes within full-length antigens, to be administered with an immune-stimulating adjuvant, may be a productive path towards efficacious vaccine development.
In an effort to determine whether two specific domains of the transferrin-binding proteins could elicit protective antibodies, we made genetic chimeras linked to the non-toxic A2 subunit of cholera toxin. Native cholera toxin is an AB5 exotoxin composed of one catalytic A subunit (CtA) and 5 surface binding B subunits (Ctb) [21]. The A and B subunits spontaneously combine in a non-covalent fashion in the periplasm to form the holotoxin [22]. The 5 individual B subunits combine to form a ring-like structure (Ctb). The non-toxic A2 domain of CtA passes through the central pore of the B subunit which allows for the tethering of the activity domain to Ctb [21]. Previous investigators have demonstrated that the replacement of the toxic A1 moiety of CtA with heterologous proteins genetically fused with the A2 subunit, allowed for production of holotoxin-like chimeras [23–29]. Employing this approach for TbpA, we focused on the surface-exposed loop 2 (L2). L2 has a predicted molecular mass of 9 kDa, and the sequence is well conserved among gonococcal isolates [12]. For TbpB, we focused on the N-terminal transferrin-binding domain (NB), which was demonstrated to be the smallest truncation that retained the ability to bind transferrin by Western blot [13]. Portions of this domain are also relatively well conserved among gonococcal isolates [13].
Because L2 is relatively small in size, and from a protein that is not especially immunogenic [19], we constructed a double genetic chimera with this peptide. The strategy we employed expressed NB and L2 together in anticipation that the larger NB would be more immunogenic and could augment antibody responses to L2. To this end, we genetically linked the L2 region in frame, immediately downstream of NB to make an NB-L2 chimera. This approach also afforded us the opportunity to determine what effects the inclusion of both epitopes would have on bactericidal killing and growth inhibition compared to mice immunized with NB only. We immunized mice intranasally and parenterally with the chimeric proteins and demonstrated that both chimeras were immunogenic, eliciting Tbp-specific serum antibodies. Furthermore, both chimeras induced bactericidal and growth inhibitory antibodies. This study demonstrates the feasibility of using epitopes instead of full-length Tbps in eliciting protective immune responses.
Materials and methods
2.1. Bacterial strains and growth conditions
The bacterial strains used in this study are listed in Table 1. E. coli strains were cultured using Luria-Bertani (LB) agar or LB broth (Difco) containing either ampicillin (100–200 µg/mL) or kanamycin (25 µg/mL). Gonococci were maintained on plates containing GC medium base (Difco) plus Kellogg’s supplement I [30]and 12 µM Fe(NO3)3. Agar plates were cultured at 37°C in a 5% CO2 atmosphere. To induce iron stress, gonococci were grown in CDM (a chemically defined medium) [31], which was pretreated with Chelex-100 (BioRad) to remove residual iron. As an alternative, GCB broth was employed with the addition of 100 µM Desferal (desferroxamine mesylate; Sigma). Liquid gonococcal cultures were grown at 35°C in 5% CO2, with shaking at 200 rpm. For flow cytometry analysis, bactericidal assays and growth inhibition measurements, gonococci were iron stressed by growth on GCB agar plus Kellogg’s supplement I and 5–10 µM Desferal, which induces iron stress and Tbp expression (data not shown).
Table 1.
Bacterial strains used in this study
| Strain | Description | Source or ref. |
|---|---|---|
| E. coli | ||
| BL21 (DE3) | F- ompT hsdSB (rB−mB−) gal dcm (DE3) | Novagen |
| C41 (DE3) | F- ompT hsdSB (rBB−mB−) gal dcm (DE3) uncharacterized derivative of BL21 (DE3) | Avidis |
| TOP 10 | F− mcrA Δ(mrr-hsdRMS-mcrBC)Φ80lacZΔM15 ΔlacX74 recA1 araD139Δ(ara-leu)7697 galU galK rpsL (StrR) endA1 nupG | Invitrogen |
| NovaBlue | endA1 hsdR17 (rk12−mk12+) supE44 thi-1 recA1 gyrA96 relA1 lac [F’ proA+B+lacIq ZΔM15∷Tn10 (Tcr)] | Novagen |
| HB101 | supE44 hsdS20 (rB−mB−) recA13 ara-14 proA2 lacY1 gaIK2 rpsL20 xyl-5 mtl-1 | [51] |
| N. gonorrhoeae | ||
| MCV601 | Lbp−[lbpB∷Ω (Strr)], derivative of FA19 | [52] |
| MCV602 | TbpA− Lbp− [tbpA∷mTn3(CMr)lbpB∷Ω(Strr)], derivative of FA19 | [52] |
| FA19 | Wild-type strain; serovar PorB1A-1 | [53] |
| MS11 | Wild-type strain; serovar PorB1B-9 | [54] |
| FA1090 | Wild-type strain; serovar PorB1B-3 | [55] |
| UU1008 | Wild-type strain | [13] |
| Pgh3-2 | Wild-type strain | [13] |
| 4102 | Wild-type strain | [13] |
| 4121 | Wild-type strain | [13] |
| 4125 | Wild-type strain | [13] |
| 4141 | Wild-type strain | [13] |
| 4146 | Wild-type strain | [13] |
| 4178 | Wild-type strain | [13] |
| 4196 | Wild-type strain | [13] |
| 4134 | Wild-type strain | [13] |
2.2. Flow cytometric analysis of binding of the L2-specific sera to the gonococcal surface
All buffers were filtered through a 0.22-µm filter (Millipore) to remove particles that could interfere with flow cytometric analysis. Gonococcal strains MCV601 (Tbp+/Lbp−) and MCV602 (TbpA−/Lbp−) (see Table 1) were incubated overnight on GCB agar plates containing Kellogg’s supplement I [30], and 12 µM Fe(NO3)3 at 37°C in a 5% CO2 atmosphere. Single colonies were passaged onto GCB plates supplemented with 10µM Desferal to induce iron stress. Bacteria were harvested into PBS + 0.05% Saponin (Sigma) to a density of approximately 2 × 108 CFU/mL. One mL aliquots of the cell suspension were spun down at 10,000 × g for 2 min and the pellets were washed twice with PBS + 0.05% Saponin. Bacteria were fixed with 1% paraformaldehyde in PBS for 30 min at room temperature while protected from light. Fixed cells were washed twice with PBS and resuspended in PBS + 0.1% IgG free BSA (Sigma) and incubated for one hour at RT. After two washes with the same buffer, cells were resuspended in L2-specific antisera [32] at the appropriate dilution in PBS + 0.1% BSA and incubated for one hour at RT. Following one wash with the same buffer, bacteria were incubated with an Alexa-488 conjugated goat anti-rabbit secondary antibody (Molecular Probes) for 30 min at RT. After one wash with the same buffer, cells were resuspended in one mL of buffer and filtered through a 35 µm nylon mesh to remove any flocculent debris. Antigen-antibody binding was measured by flow cytometry as median fluorescence intensity with a Coulter EPICS XL-MCL flow cytometer, with four-decade logarithmic amplification. Approximately 30,000 events were counted with events triggered on a side scatter (SC) with a threshold of 1.
2.3. Western blot assays
Western blots were performed using iron-stressed gonococci, or purified recombinant proteins transferred onto a nitrocellulose membrane (Schleicher & Schuell). For detection of TbpA L2, blots were probed with rabbit antisera raised against purified recombinant TbpA [33]. NB was detected with rabbit antisera raised against recombinant TbpB (kindly provided by Christopher Thomas and P. Frederick Sparling). Ctb was detected using rabbit anti-cholera toxin sera (Sigma). Blots were developed with nitroblue tetrazolium-5-bromo-4-chloro-3-indolyl phosphate (BCIP).
2.4. Construction of expression plasmids
The NB-Ctb chimera was constructed by PCR amplification of a region encoding the N-terminal binding domain (NB) of tbpB [13] using genomic DNA from strain FA19 as template. The forward primer, oVCU231, (CCATGGCCCTGGGCGGAGGCGGCAGTTTCG) contained an NcoI site (shown in bold), and encoded the N-terminus of the mature tbpB from amino acid +2. The reverse primer, oVCU232 (CTCGAGGTCGACAACCAGTCGGGTAGCG), contained an XhoI site (shown in bold), and amplified the region encoding the C-terminus of the previously described transferrin binding domain [13]. The resulting PCR product was ligated into pCTΔA1 [23] creating the expression plasmid pVCU720. The NB-L2-Ctb expression plasmid was constructed by PCR amplification of the region encoding surface exposed loop 2 of TbpA from genomic DNA of gonococcal strain FA19. The forward primer, oVCU319 (CTCGAGGGATCCCGCACCGGGCGGCACGCG), contained an XhoI (shown in bold) site with a nested BamHI site (shown bolded and underlined). The reverse primer, oVCU230 (CTCGAGCGGATCGGCGAGGAAGCGGTTGG), contained an XhoI site (shown in bold). These primers amplified the region encoding loop 2 of TbpA [32]. The resulting PCR product was ligated into the XhoI site of pVCU720 creating the expression plasmid pVCU724. The Ctb expression vector pVCU721 was constructed by PCR amplification of the mature ctb gene from plasmid pCTΔA1 [23]. The forward primer, oVCU238 (TGGCCACACCTCAAAATATTACTGATTTGTGTG) contained an MscI site (shown in bold) and amplified the mature ctb gene. The reverse primer, oVCU310 (CTCGAGATTTGCCATACTAATTGCGGCAATCG), contained an XhoI site (shown in bold) and amplified the 3’ end of the ctb gene just prior to the stop codon. The PCR product was ligated into pET-22b(+) (Novagen), which resulted in a 6X histidine tag being fused immediately downstream of the ctb gene. To construct the NB-Ctb(His) and NB-L2-Ctb(His) expression constructs, pVCU720 and pVCU724 were digested with NdeI. Digestion with NdeI liberated fragments that encoded either NB-A2 (pVCU720) or NB-L2-A2 (pVCU724) and a partial fragment of the ctb signal sequence. These gene fragments were inserted into an Nde I site in pVCU721. This created the expression plasmids pVCU722 and pVCU725. The E. coli expression host for these recombinant plasmids was C41 (DE3) (Avidis).
2.5. Sequencing the gene fragments that encode NB and L2 from gonococcal strain MS11
The regions encoding NB and L2 from gonococcal strain MS11 were amplified from genomic DNA employing Platinum Taq High-Fidelity polymerase (Invitrogen) and the primers described above. PCR products were cloned into pCR2.1 (Invitrogen) and at least six individual clones of each PCR product were sequenced. DNA sequence analysis was conducted by the VCU Nucleic Acids core facility. Amino acid alignments were constructed with Vector NTI software. Accession numbers corresponding to the MS11 partial gene sequences are as follows: NB-encoding region, EF547129; L2-encoding region, EF547130.
2.6. Recombinant protein expression and purification
Recombinant E. coli strains containing the chimeric plasmids, pVCU722 (NB-Ctb(His)) and pVCU725 (NB-L2-Ctb(His)), were grown in 5mL starter cultures using 2x YT medium (1.6% tryptone w/v, 1% yeast extract w/v, and 0.5% NaCl w/v) supplemented with 1% glucose and 200 µg/mL of ampicillin. After starter cultures became turbid, they were used to inoculate 1L cultures of 2x YT and allowed to grow at 37°C with shaking at 225 rpm until the OD600 reached ~1.5. Protein expression was induced by the addition of 0.25 mM IPTG followed by incubation at 27°C for 16–18 hours. Following expression, cultures were centrifuged for 15 min at 4,000 × g and pellets were stored at −80°C.
For chimera purification, proteins were isolated from periplasmic extracts. Cell pellets were resuspended in ice cold periplasmic extraction buffer (100 mM Tris-HCl, pH 8.0, 20% sucrose, 5 mM EDTA, and 0.5 mg/mL lysozyme) and incubated on ice for 20 min to allow for the release of periplasmic contents. Following incubation on ice, spheroplasts were pelleted by centrifugation at 12,000 × g and discarded. Periplasmic contents were subjected to ammonium sulfate precipitation where the majority of the chimeric proteins precipitated between 30–60% saturation. Following centrifugation, the precipitated material was resuspended in phosphate buffer containing 50mM NaH2PO4, 300mM NaCl, and 20mM imidazole, pH 8.0. The resuspended proteins were batch-bound to nickel resin for one hour at 4°C. Following binding, the resin was loaded onto a disposable column and washed with 20-bed volumes of 50mM NaH2PO4, 300mM NaCl, and 20mM imidazole, pH 8.0, and eluted using a buffer containing 50mM NaH2PO4, 300mM NaCl, and 250mM imidazole, pH 8.0. Purified proteins were dialyzed twice against a 1000-fold excess volume of PBS and stored at −80°C.
2.6. GM1 ganglioside ELISA
Purified chimeras were bound to GM1 ganglioside in an ELISA format and complexes with binding competent Ctb pentamers were confirmed to contain NB and L2 with specific antibodies [19].
2.7. Mouse immunizations and sample collection
Female BALB/c mice, 8 weeks old were purchased from Charles River Laboratories (Wilmington, MA). The mice were housed in microisolator cages and were under the care and supervision of the Division of Animal Resources. The protocols were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee. At the start of the study the mice were ca. 12 weeks old. Groups of five were immunized either intranasally (IN) or subcutaneously (s.c.) with one of the Tbp chimeras (refer to table 2 for immunization details). All groups were immunized 3 times at 10 day intervals. Twenty-five days following the third immunization, the mice were all boosted intraperitoneally (IP) with 20 µg of total protein. All groups received two IP boosts 10 days apart. Sera and vaginal washes were obtained as described previously [19], and collected on days 0, 18, 28 (secretions only), 35, 63, and 82.
Table 2.
Immunization groups
| Group (immunization routea) | Immunogen | Amt administeredb (µg) |
|---|---|---|
| NB-Ctb (IN/IP) | NB-Ctb complex | 20 |
| NB-L2-Ctb (IN/IP) | NB-L2-Ctb complex | 20 |
| NB-L2-Ctb (s.c./IP) | NB-L2-Ctb complex | 20 |
| Control (IN/IP) | Buffer | 0 |
Mice were immunized three times either intranasally (IN) or subcutaneously (s.c.), followed by 2 boosts given intraperitonealy (IP).
Groups of mice (n = 5) were immunized three times at 10 day intervals. Twenty-two days following the third vaccination, the mice were boosted two times at 10-day intervals.
2.8. Quantitative ELISAs
Sera and vaginal washes were assayed for antibodies specific for TbpA, TbpB, or total IgG and IgA (vaginal washes only) as described previously [19, 33]. Capture and alkaline phosphatase-conjugated goat anti-mouse antibodies were purchased from Southern Biotechnology Associates (Birmingham, Al). The standard curve was generated using a mouse reference serum (Bethyl Laboratories), and unknown samples were interpolated from the standard curve using the four-parameter logistic model. Vaginal antibody levels are presented as percentage of total corresponding immunoglobulin isotype to compensate for sampling error, sample-to-sample dilution differences, and isotype fluctuations due to the mouse estrus cycle [34]. Antibody amounts were transformed to logarithms to normalize the distribution and variance of the data. For presentation purposes the log transformed data were back transformed to arithmetic values to generate geometric means ×/÷ SD.
2.9. Serum bactericidal assays
Serum bactericidal assays were performed essentially as previously described [19]. Day 63 sera were pooled by group and heat inactivated at 56°C for 30 min. Gonococcal strains were plated from freezer stocks onto plates containing GC medium base plus Kellogg’s supplement I and 5 µM desferal to induce iron stress. Following 16–18 hours growth at 37°C with 5% CO2, isolated colonies were picked and suspended in 37°C Gey’s balanced salt solution (Sigma) containing 0.1% gelatin and 5 µM desferal (GBSS+G+D) to an OD600 of 0.2 (0.23 for strain MS11). The suspension was serially diluted to 10−5 in GBSS+G+D. The diluted inoculum (80µl) was placed in a prewarmed (37°C) microtiter plate containing 10µl of the appropriate serum samples diluted in GBSS+G+D. The plate was incubated at 37°C and 5% CO2 for 15 min., then 10 µl of pooled normal human serum (Quidel Corp.) was added and incubated again as above for 45 min. Because strain MS11 was moderately serum sensitive, 5% human serum was used, and incubated as above for 15 min. Following incubation, cell viability was determined by plating onto GC medium base plus Kellogg’s supplement I and 12.5 µM ferric nitrate. Following 24 hour incubation as above, colonies were enumerated. Reported titers were calculated as the reciprocal of the dilution that resulted in > 50% killing as compared to CFU detected in the presence of the sham-immunized, control sera at the same dilution. Each assay included sera from animals immunized with PBS and sera from vaccinated animals. Assays were performed at least in triplicate.
2.10. Growth inhibition assays
Gonococcal strains were plated from freezer stocks onto GCB plates plus Kellogg’s supplement I and 5µM desferal to induce iron stress. Plates were incubated at 37°C in a 5% CO2 atmosphere for approximately 18 hours. Isolated colonies were removed from the plate and resuspended in iron free CDM to an OD600 of approximately 0.15. Eighty-seven microliters of this suspension was loaded into a sterile 96- well flat-bottomed microtiter plate. Iron saturated transferrin was then added to a final concentration of 7.5 µM and 10 µL of pooled vaginal wash was added. Following an initial OD600 reading in a microplate reader, the plates were incubated at 37°C in a 5% CO2 atmosphere with shaking at 225 rpm. Every two hours, the optical density was measured as above. All samples were tested in duplicate, and each assay was conducted 3 separate times. The number of viable bacteria at t=0 min. was 2 × 107 for FA19 and 8 × 106 for FA1090.
3. Results
3.1. Rationale for selection of epitopes for inclusion in a chimeric vaccine
The impetus for incorporation of L2 of TbpA in the subunit vaccine was based on immunogenicity and surface exposure results obtained with L2-specific rabbit antiserum (Fig. 1). An L2-specific antiserum was previously generated against 83 amino acids of mature TbpA, from R198 to P280 [32]. As shown in Figure 2A, Western blot analysis with the L2-specific serum indicated that antibodies reactive with L2 recognized every gonococcal strain tested but did not react with the negative control strain lacking TbpA (Fig. 1A., lane 1). We also conducted flow cytometric analysis using pre-immune and post-immune sera to establish surface binding of the L2-specific antiserum. As shown in Fig. 1B, robust surface binding of the post-immune sera to the TbpA+ strain was detected, as compared to the pre-immune sera. Specificity was demonstrated by reduced binding of the post-immune antisera against a mutant strain that lacks TbpA (Fig.1C). Surface exposure of the L2 epitope was demonstrated in a previous study wherein an HA epitope insertion into the L2 domain was also surface exposed in whole gonococci [35]. We have previously noted that the sequence comprising L2 of TbpA was well conserved among gonococcal strains [12] and in the current study, this observation was extended to include L2 from gonococcal strain MS11 (Fig. 2). Cumulatively these data indicate that the L2 region of TbpA is surface exposed, immunogenic, and conserved among diverse gonococcal isolates [36, 37]. These results stimulated our interest in including this epitope of TbpA in a subunit vaccine.
Fig. 1.
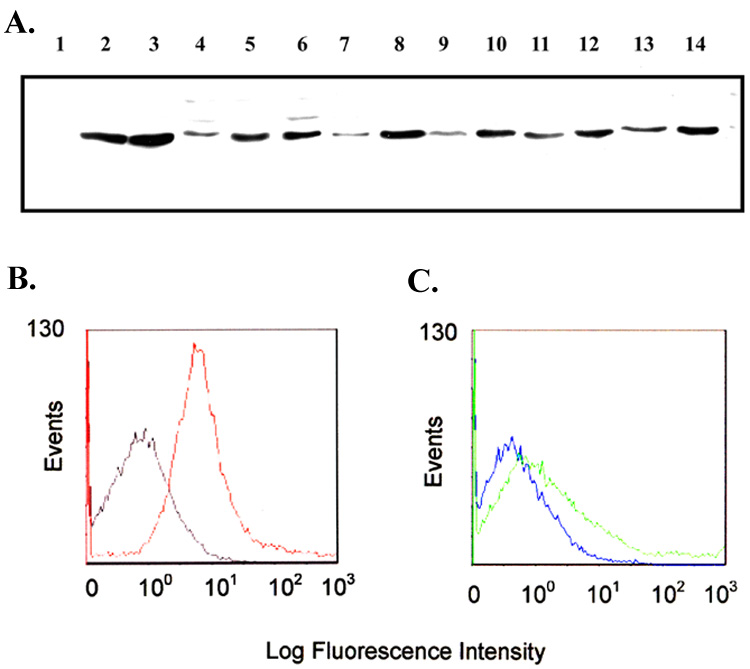
Immunoblot blot and flow cytometric analysis of L2 epitope sera. Panel A. Immunoblot containing whole-cell lysates of iron-stressed gonococci probed with the L2 peptide sera. Lanes contain proteins from the following strains: 1, MCV602; 2, FA19; 3, FA1090; 4, UU1008; 5, Pgh3-2; 6, DC966; 7, 4102; 8, 4121; 9, 4125; 10, 4141; 11, 4146; 12, 4178; 13, 4196; 14, 4134. Panel B. Flow cytometry histograms showing surface binding of the L2 epitope sera to gonococcal strain MCV601 (TbpA+). Gray line represents binding of pre-immune serum. Red line represents binding of post-immune serum. Panel C. Flow cytometry showing lack of surface binding of L2 epitope sera to strain MCV602 (TbpA−). Blue line represents pre-immune sera, and green line represents post-immune sera.
Fig. 2.
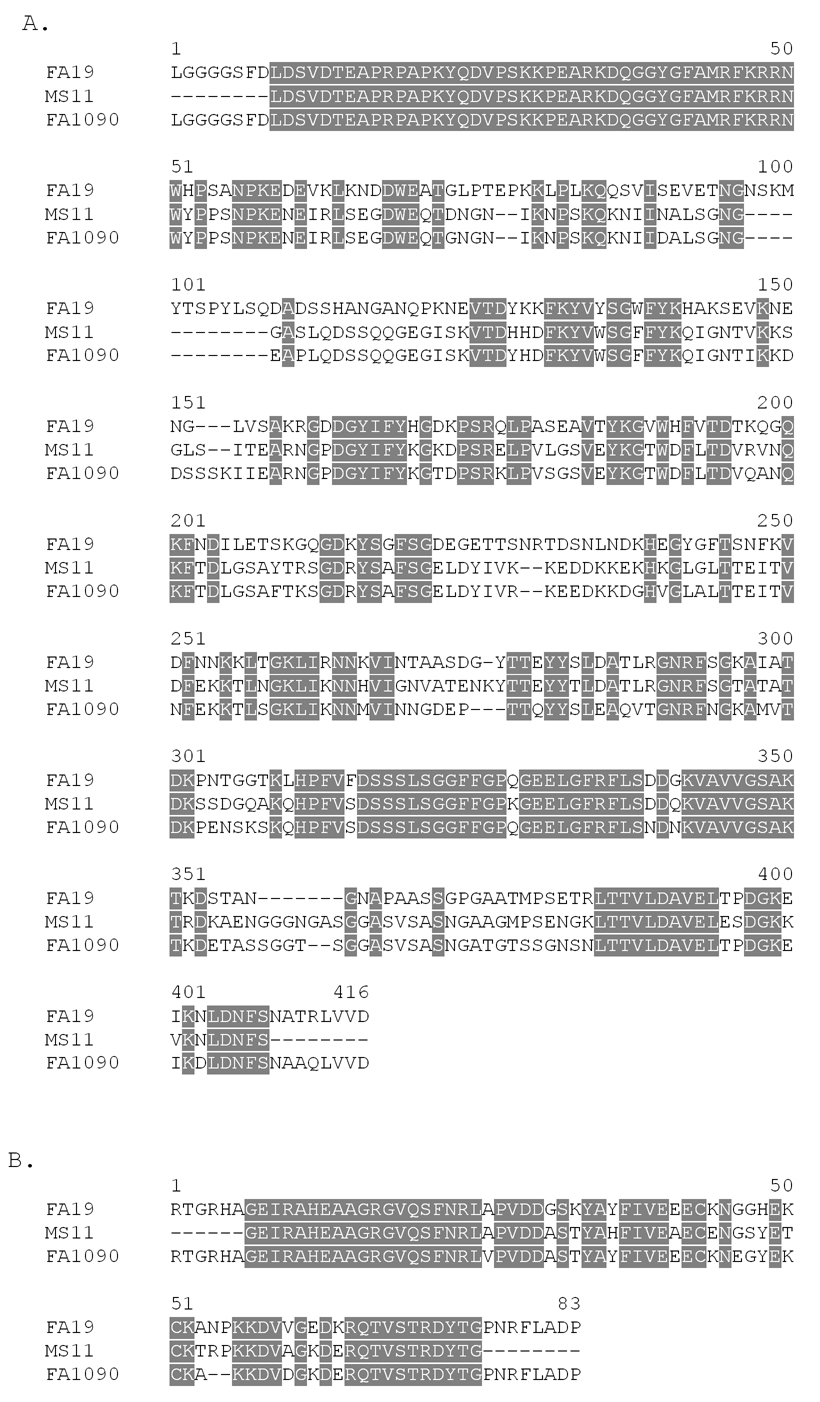
Sequence alignments of the NB and L2 domains. Panel A. Amino acid sequence alignment of the NB domain of TbpB from strains FA19, MS11, and FA1090. Panel B. Amino acid sequence alignment of the L2 domain of TbpA from strains FA19, MS11, and FA1090. The MS11 sequences, determined as part of this study, lack the terminal residues of NB and L2 domains since the regions encoding these amino acids were amplified with FA19-specific primers.
The NB region of TbpB encompasses 405 amino acid residues, from L2 to D406. Anti-peptide serum generated against a portion of this region was broadly cross-reactive against a panel of diverse gonococcal and meningococcal isolates [13]. The peptide sequences of two regions within the NB domain were found to be quite well conserved among the Neisseriae [13] . These peptide regions were also well conserved in the NB domain of MS11 (Fig. 2). Moreover, the NB domain was capable of binding transferring after SDS-PAGE and electroblotting [13]. These factors prompted us to include the amino-terminal NB region of TbpB in the subunit vaccine.
3.2. Construction of the Tbp-Ctb chimera expression vectors
Initially, the sequence encoding the NB domain of tbpB was PCR amplified and inserted into the previously described expression vector pCTΔA1 [23] creating the expression plasmid pVCU720 (Fig. 3A). To make the double NB-L2 chimera, the L2 domain of TbpA [12] was PCR amplified and inserted into the XhoI site of pVCU720 creating the expression plasmid pVCU724 (Fig. 3B). Initial experiments suggested that expression, following addition of IPTG, from both pVCU720 and pVCU724 was toxic to E. coli strain BL21 (DE3). We reasoned that this was likely due to the native signal sequence of Ctb interfering with export in E. coli [38]. In order to circumvent the toxicity issues, the regions encoding NB and NB-L2 were excised from their respective plasmids using NdeI (Fig. 3). These DNA fragments were inserted into the unique NdeI site in the Ctb expression vector, pVCU721. This plasmid contained the mature ctb gene immediately downstream of the pelB leader sequence, which mediates export to the periplasmic space in E. coli. The resulting hybrid expression plasmids, pVCU722 and pVCU725 (Fig. 3A and 3B), encoded Ctb proteins fused to a 6X-histidine tag on the C-terminus and a pelB leader sequence on the N-terminus. In addition, these fusion plasmids encoded NB fused to the non-toxic A2 domain (pVCU722) or NB and L2 fused to the A2 domain (pVCU725). In the periplasm, the A2 domain enabled the assembly of toxin-like complexes of NB or NB-L2 antigens with binding-competent Ctb pentamers. E. coli strains containing both pVCU722 and pVCU725 showed much less toxicity than their predecessors upon induction with IPTG.
Fig. 3.
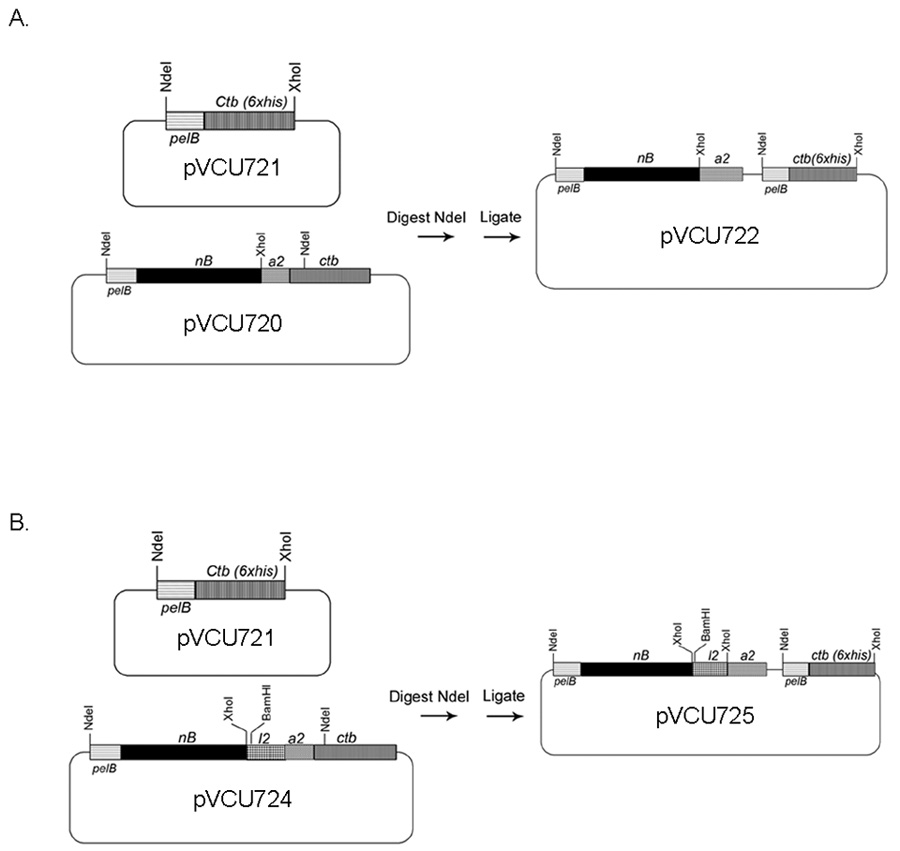
Construction of the NB-Ctb(His) and NB-L2-Ctb(His) expression vectors. Panel A. The expression plasmid pVCU720 was digested with NdeI liberating a DNA fragment encoding the PelB leader sequence fused to the NB fragment and the Ctb A2 fragment. This fragment was inserted into the Ctb(his) expression vector, pVCU721, which was similarly digested with NdeI, creating the NB-Ctb(his) expression plasmid, pVCU722. Panel B. The expression plasmid pVCU724 was digested with NdeI liberating a DNA fragment encoding the following, in order: PelB leader sequence fused to the NB region, followed by the L2 domain, and the A2 region. The NdeI fragment was inserted into the Ctb(his) expression vector pVCU721, which was similarly digested with NdeI, creating the NB-L2-Ctb(his) expression plasmid pVCU725. Plasmids and genes are not drawn to scale.
3.3. Purification of the Ctb(his) chimeras
Both chimeric antigens were isolated from periplasmic extracts and purified by nickel affinity resin. Both NB-A2 and NB-L2-A2 fusion proteins formed complexes with Ctb pentamers, which were resistant to SDS dissociation by SDS-PAGE (Fig. 4A). The Ctb pentamer migrates at a molecular mass of approximately 52 kDa as demonstrated in the non-heated, non-reduced samples. After boiling, the pentamer is dissociated and the monomers migrate at a molecular mass of approximately 12 kDa (Fig. 4A). Although the Ctb pentamer is resistant to the effects of SDS, the A subunit dissociates from the B subunit upon SDS-PAGE. The full activity subunit (CtA) of holo-cholera toxin has a predicted molecular weight of approximately 27 kDa, and migrated accordingly (Fig. 4A, CT control lanes). The NB-A2 and NB-L2-A2 chimeras have predicted molecular masses of 53 and 61 kDa, respectively. As detected by SDS-PAGE, the chimeric NB-A2 peptides ran as doublets of similar apparent molecular mass (Fig. 4A). The smaller product likely results from a secondary start site within TbpB, as has been demonstrated previously [33]. High molecular mass species were apparent in Western blots using TbpB- and TbpA-specific antiserum (Fig. 4B). The anti-TbpA serum was also highly reactive with a band of ~22 kDa, although no band of this size was apparent by Coomassie blue staining (Fig. 4A). The molecular mass of this species is similar to that predicted for L2-A2 without NB (~14 kDa).
Fig. 4.
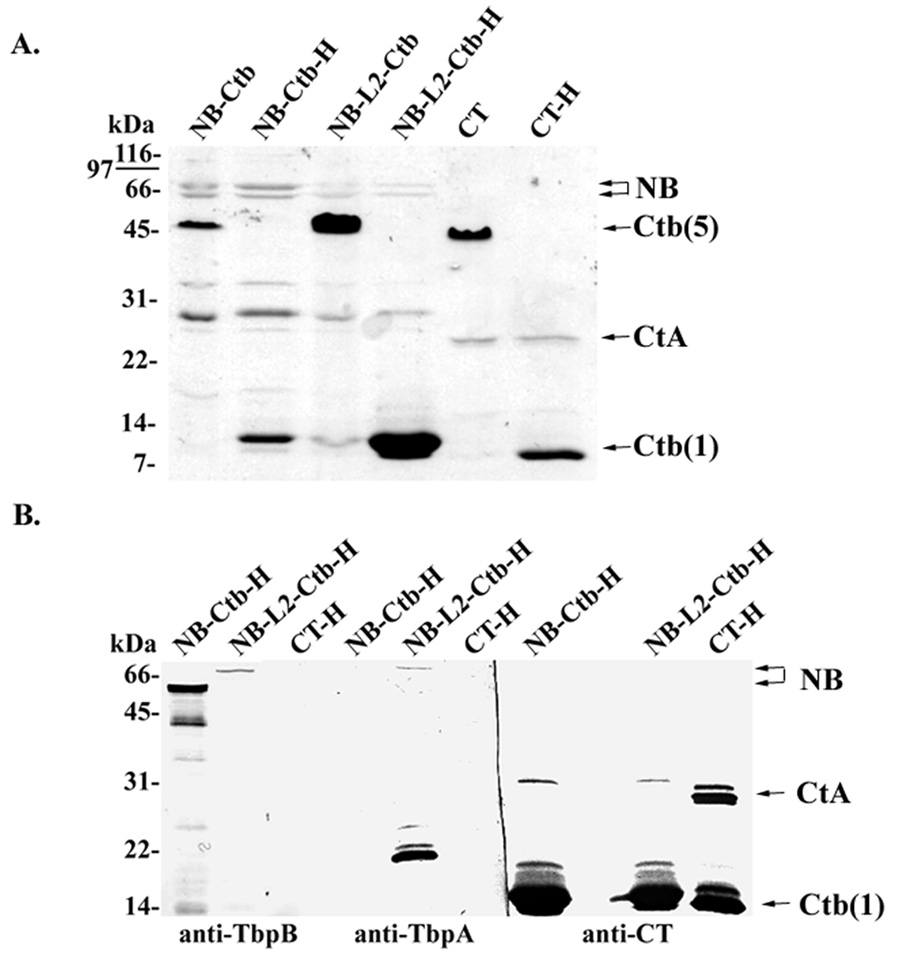
SDS-PAGE and Western blot analysis of purified Ctb chimeras. Panel A. Coomasie-stained SDS-PAGE of purified chimeric antigens. Lanes labeled NB-Ctb contained NB-A2 fusions complexed with Ctb; lanes labeled NB-L2-Ctb contained NB-L2-A2 fusions complexed with Ctb; lanes labeled CT contained commercially-available holo-cholera toxin as a control. Samples were separated by SDS-PAGE either without prior reduction and heating, or with reduction and heat (H). Panel B. Western blot analysis of purified chimeric antigens and CT using antisera specific for TbpB, TbpA, and CT. Lanes are labeled as indicated for panel A except that all samples were reduced and heated before separation by SDS-PAGE.
Both NB-A2 and NB-L2-A2 chimeras were relatively unstable, resulting in various breakdown species as visualized in both SDS-PAGE and Western blot analyses (Fig. 4). This result was not unexpected, as TbpB and TbpB peptides have been previously shown to be unstable, producing breakdown products following SDS-PAGE [39]. The NB-L2-A2 chimera however did appear to be more stable as there were fewer breakdown species as demonstrated by Western blot analysis (Fig. 4B). It is possible that the addition of the L2 peptide between the NB and A2 domains stabilized the NB domain. This however is difficult to determine as the NB-L2-A2 domain was poorly assembled resulting in higher free Ctb concentrations and lower NB-L2-A2 concentrations as compared to the NB-A2-Ctb counterpart (Fig. 4). Therefore the fewer breakdown products seen with the NB-L2-A2 preparation could have been an artifact of a lower concentration of NB-L2-A2 compared to the NB-A2. The NB-Ctb chimera appeared to have fewer free Ctb molecules as it had similar band intensities as seen in CT control lanes (Fig. 4A). Issues with stoichiometry are complicated however due to the propensity of NB to breakdown following SDS-PAGE, which could falsely suggest poor assembly. Assembly of binding-competent Ctb pentamers with associated NB and L2 was verified by GM1 ganglioside ELISA (data not shown).
3.4. Serum antibody responses
Four groups of five female BALB/c mice were immunized for this study (Table 2). At various times after immunization of each group, serum antibody levels were measured by quantitative ELISA against full-length TbpA and TbpB. Following the three initial immunizations, antibody responses to TbpB in all groups immunized with the chimeras were low (Fig. 5A). The TbpA-specific antibody levels detected in the groups immunized with the NB-L2-Ctb chimera were even lower (Fig. 5B). Because low serum antibody responses were detected after three IN immunizations, we boosted all the immunized groups intraperotoneally (IP). IP immunization was recently shown to boost serum and vaginal antibody responses following IN priming immunizations [40]. This IN/IP vaccination strategy significantly enhanced antibody titers to TbpB in all immunized groups. Antibody levels ranged from 50–100 fold greater than the antibody amounts measured before IP boost (Fig. 5 days 18 and 35 compared to days 63 and 82).
Fig. 5.
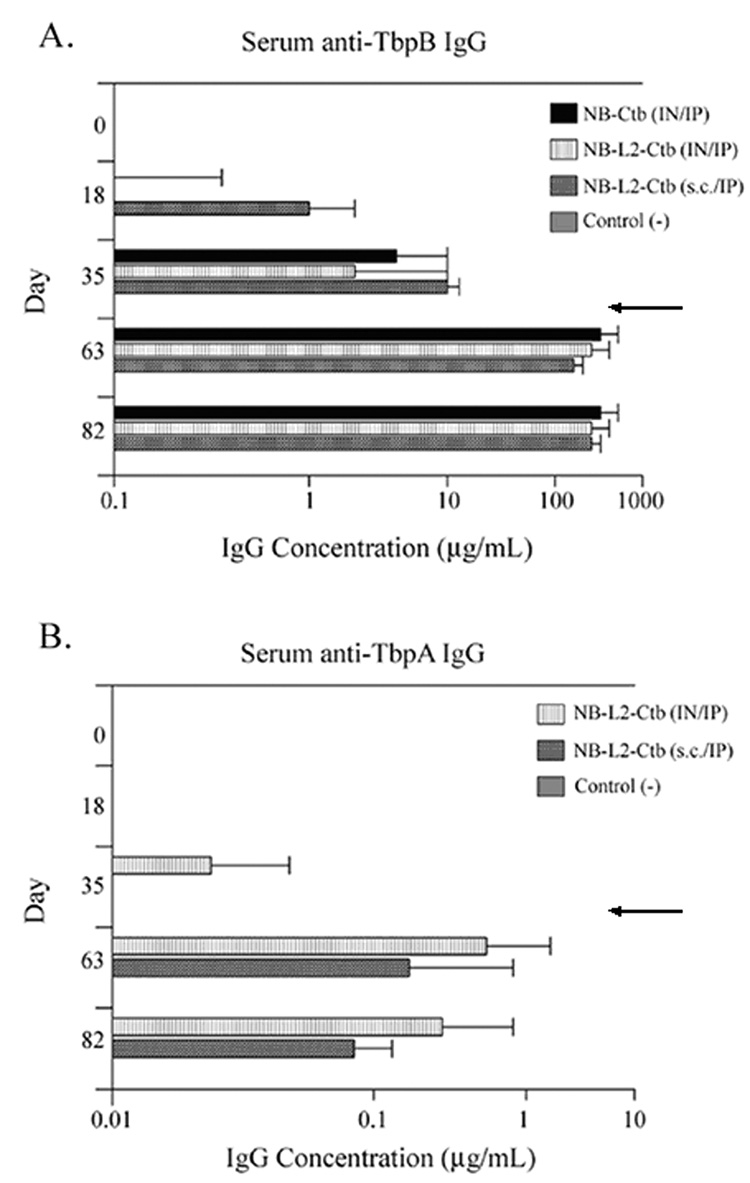
Serum IgG levels specific for TbpB and TbpA. Panel A. Serum IgG levels specific for TbpB detected on days 0, 18, 35, 63, and 82. Panel B. SerumIgG levels specific for TbpA detected on days 0, 18, 35, 63, and 82. Results are expressed as the geometric mean of antibody titers ×/÷ standard deviation. For all immunization groups n = 5 with the exception of the group immunized with NB-L2-Ctb (s.c.) where n = 4 on days 63 and 82. Arrows indicate the time at which all animals were boosted IP. Note: the axes of panels A and B are different.
In addition to enhancing antibody titers against TbpB, IP immunization increased antibodies directed towards TbpA in all the groups immunized with chimeras containing L2. Although all groups immunized with the NB-L2 chimeras elicited TbpA-specific antibodies, these levels were still much lower than the antibody levels measured against TbpB. Comparison of serum antibody levels in all immunized groups on days 63 and 82 revealed a decline in TbpA-specific antibody levels (Fig.5B).
3.5. Vaginal antibody responses
Similar to observations made with the sera, vaginal antibody levels to TbpB in the immunized groups were very low prior to the IP boosts (Table 3). Before IP boosting, the IN primed groups had low but detectable levels of TbpB-specific IgA (Table 3, day 28). Vaginal IgG levels were undetectable in either of the IN-immunized groups, but were detected in the group immunized s.c. (Table 3, day 28).
Table 3.
Vaginal antibody levels specific for TbpB detected by ELISAa
| Day 28b | Day 63c | |||
|---|---|---|---|---|
| Immunization | ||||
| IgA | IgG | IgA | IgG | |
| NB-Ctb (IN/IP) | 0.4 ×/÷ 4.4 | 0 | 0.5 ×/÷ 3.0 | 7.1 ×/÷ 3.4 |
| NB-L2-Ctb (IN/IP) | <0.1d | 0 | 0.6 ×/÷ 5.3 | 10.8 ×/÷ 1.7 |
| NB-L2-Ctb (s.c./ IP) | 0 | 5.8 ×/÷ 3.5e | 0.7 ×/÷ 3.8f | 12.8 ×/÷ 1.3f |
Data are expressed as the geometric mean of the percentage (specific antibody/total antibody) ×/÷ standard deviation.
Day 28 is 7 days after final IN prime.
Day 63 is 7 days after final boost.
Only two mice had detectable TbpB-specific antibodies.
Only three mice had detectable TbpB-specific antibodies.
n = 4.
Following the IP boosts, all of the groups had measurable TbpB-specific IgA and IgG (Table 3, day 63). Although IP immunization elicited vaginal IgA, as all groups seroconverted, the ratio of TbpB-specific to total IgA was much lower than the IgG ratio (Table 3). All mice had detectable TbpB-specific IgG on day 63, which corresponded with their increase in serum IgG levels. This result was not unexpected as vaginal IgG levels are considered to result from serum transudation [41]. In contrast to the situation with NB, TbpA-specific antibodies in vaginal secretions of L2 immunized mice were below the limits of detection for this assay.
3.6. Serum bactericidal activity
In order to determine whether the chimeric antigens could elicit protective antibodies, we performed in vitro bactericidal assays using pooled human sera as a complement source. We pooled day 63 sera from the mice in each group and determined relative bactericidal activities against homologous and heterologous gonococcal strains. All the sera analyzed had similar bactericidal titers against the homologous strain (FA19) and one heterologous strain (MS11; Table 4). The other heterologous strain tested, FA1090, was the most resistant to bactericidal killing by all the sera tested. Surprisingly, the groups immunized with NB-L2-Ctb had detectable bactericidal antibodies against this relatively resistant strain (Table 4). However, no detectable bactericidal antibodies were identified within the group of mice immunized with NB-Ctb, even though this immunization group had the highest amount of TbpB-specific antibody on day 63 (Fig. 5B).
Table 4.
Serum bactericidal activities of sera collected on day 63
| Serum bactericidal titera for strain: | |||
|---|---|---|---|
| Immunization group | |||
| FA19 | MS11 | FA1090 | |
| NB-Ctb (IN/IP) | 200 (74% ± 9.2) | 400 (79% ± 1.4) | >25b |
| NB-L2-Ctb (IN/IP) | 200 (62% ± 0.7) | 400 (66% ± 21.2) | 50 (60% ± 10.6) |
| NB-L2-Ctb (s.c./IP) | 200 (80% ± 8.5) | 400 (58% ± 9.9) | 100 (70% ± 3.5) |
Titers were calculated as the reciprocal of the dilution that gave >50% killing compared to the sham-immunized control sera. The average percent killing determined from duplicate assays ± standard deviation is shown in parentheses.
Less than 50% killing was achieved at a dilution of 1/25. Lower dilution factors were not tested.
3.7. Vaginal antibodies from mice immunized with chimeras inhibited gonococcal growth in vitro
Having demonstrated bactericidal activity of serum antibodies, we next evaluated whether antibodies obtained from vaginal washes could impede the growth of the gonococcus in vitro. In media containing transferrin as the sole iron source, growth of the homologous gonococcal strain (FA19) was inhibited by pooled vaginal wash samples from the NB-Ctb group and the NB-L2-Ctb group (Fig. 6A). The pooled wash samples from the NB-Ctb immunized group did not inhibit the growth of the heterologous strain (Fig. 6B); however, the pooled washes from the NB-L2-Ctb group were growth inhibitory for FA1090 (Fig. 6B). These data are somewhat incongruent with the fact that we were unable to detect vaginal TbpA-specific antibodies in any of the vaginal washes. Therefore it is possible that the growth inhibition detected in the presence of the NB-L2-Ctb vaginal washes was actually due to higher TbpB-specific antibody concentrations detected within this group of animals, as compared to the NB-Ctb immunized group (Table 5).
Fig. 6.
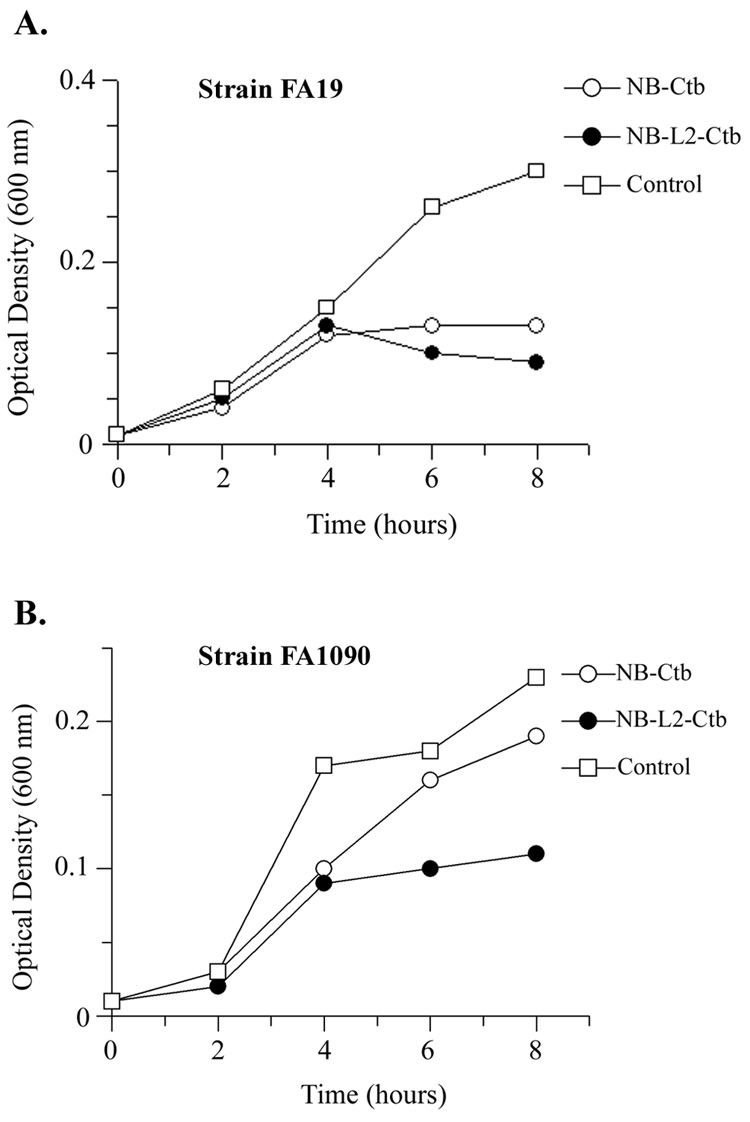
Growth inhibition of strains FA19 and FA1090 grown in the presence of day 63 vaginal wash antibodies and human transferrin as a sole iron source. Panel A. Growth inhibition of strain FA19 in the presence of either NB-Ctb or NB-L2-Ctb vaginal wash samples. Panel B. Growth inhibition of strain FA1090 in the presence of either NB-Ctb or NB-L2-Ctb vaginal wash samples. Controls were pooled vaginal wash samples from sham immunized mice obtained on day 63. Each growth curve was performed in duplicate, with optical density levels measured every 2 hours. Data are representative of three independently-conducted experiments.
Table 5.
TbpB-specific and total antibody concentrations detected by ELISA in vaginal wash samples collected on day 63
| Concentration (ng/mL)a |
||||
|---|---|---|---|---|
| Immunization | Anti-TbpB IgA | Anti-TbpB IgG | Total IgA | Total IgG |
| NB-Ctb (IN/IP) | 70 ×/÷ 6 | 78 ×/÷ 14 | 14956 ×/÷ 2 | 1100 ×/÷ 6 |
| NB-L2-Ctb (IN/IP) | 58 ×/÷ 15 | 222 ×/÷ 6 | 9114 ×/÷ 4 | 2049 ×/÷ 4 |
| Control | 0 | 0 | 33577 ×/÷ 2 | 870 ×/÷ 2 |
Data are represented as geometric means×/÷standard deviation.
4. Discussion
In this study, we demonstrate that the production of a chimeric vaccine by genetically fusing epitopes of the gonococcal Tbps to the A2 subunit of cholera toxin is feasible. The subunit chimeras complex with Ctb pentamers and result in the production of robust and protective immune responses to A2-fused antigens [23–29]. We focused our efforts on two Tbp-specific domains, the NB domain from TbpB and the L2 domain from TbpA based largely on surface exposure and strain-to-strain sequence conservation. Though TbpB is considered to be fully surface exposed [42, 43], we demonstrated L2 surface exposure by flow cytometric analysis. Because we utilized fixed instead of live cells in our surface binding studies, it is possible surface accessibility of the L2 domain by L2 antisera was due to non-native conformations of surface TbpA molecules [44]. However computer generated topology modeling of TbpA in both N. meningitidis and N. gonorrhoeae demonstrate the L2 domain to be the largest surface exposed domain [45, 46]. In N. meningitidis, L2 is predicted to be the longest surface exposed structure stretching higher than the outer membrane lipopolysaccharide [46]. This in conjunction with human studies demonstrating the existence TbpA serum-specific antibodies following carriage or infection provide evidence that the L2 epitope is unlikely to be completely hidden in the live cell [33, 47].
We demonstrated in this study that both the NB-only chimera and a double chimera comprised of both NB and L2 domains elicited biologically-functional, Tbp-specific antibodies in mice following vaccination. The immunogenicity of these chimeric antigens however was relatively low in comparison to the antibodies generated against the full-length Tbps chemically conjugated to Ctb following three intranasal immunizations [19]. Geometric mean titers to TbpB measured on day 35 in the NB-Ctb and NB-L2-Ctb immunization groups were 3.9 µg/mL and 1.2 µg/mL respectively. This is in stark contrast to the geometric mean titer of 102 µg/mL which we measured on day 35 following three IN immunizations with TbpB chemically conjugated to Ctb [19]. The poor immunogenicity in this study was likely contributed to by chimera instability, as demonstrated by breakdown products on Western blots, and dilution of antigen preparations with empty Ctb pentamers. However, after two IP boosts serum anti-TbpB titers increased dramatically in the NB-Ctb and NB-L2-Ctb groups (305ug/mL and 251 ug/mL respectively, day 63). These levels surpassed the highest levels measured to TbpB in our previous study with the group immunized IN with TbpB chemically conjugated to Ctb (102ug/mL, day 35) [19]. Besides a dramatic increase in serum TbpB IgG titers following the IP boosts, vaginal IgG antibody levels elicited against the NB domain increased dramatically as well. Vaginal IgA levels on the other hand were lower than anticipated. Although IP vaccination successfully elicited robust serum and vaginal IgG to the NB portion of the chimeras, antibody responses to the L2 epitope of TbpA were poor. The highest serum titer measured to TbpA was 0.6 µg/mL in the IN primed group (day 63). In our previous study the group immunized IN with TbpA chemically conjugated to Ctb had a maximal titer of 4.6 µg/mL measured on day 35 [19]. Serum TbpA titers were low, and vaginal anti-TbpA levels were below the limits of detection, even after five vaccinations. The explanation for this difference in immunogenicity to each of these different Tbp domains is likely two-fold. First, there is a large size difference between these two antigens. The NB domain has a predicted molecular mass of ~44 kDa, while the L2 domain is ~9 kDa. The small size of L2 could account for its relatively poor immunogenicity [48]. Second, full-length gonococcal TbpA is not as immunogenic as TbpB [19]. Thus taking a small peptide from a protein that is poorly immunogenic overall could account for the low antibody response to L2. It is also possible that the ELISA-based system for quantitation of TbpA-specific antibody underestimated the amount of functional antibody present in the serum and secretions. Antibodies capable of binding surface exposed, conformational epitopes would likely be important in biological function, but could be missed by ELISA quantitation using recombinant TbpA protein. The rationale for combining the L2 domain with the NB domain was to determine whether combining a longer, more immunogenic polypeptide (NB) with a smaller, less immunogenic peptide (L2) could enhance the immune response to the smaller peptide. In this study, combining L2 with NB in the manner described did not enhance detectable antibody levels to L2, although the biological activities of the antibodies were augmented by the presence of L2 (see below).
As a correlate of protection, we determined whether antibodies elicited against the NB or NB-L2 domains could kill heterologous or homologous gonococcal strains. We previously demonstrated the induction of cross-reactive, bactericidal antibodies following intranasal immunization using full-length TbpA and TbpB [19]. In the present study, we demonstrated that regardless of the route of immunization, and regardless of the presence or absence of the L2 epitope, bactericidal antibodies were induced against the homologous strain FA19. Immunization with the chimeras even induced bactericidal antibodies against the heterologous strain MS11, resulting in titres that were similar to those generated by full-length Tbps conjugated to Ctb [19]. Furthermore, the bactericidal titers against MS11 were 2-fold higher than those against the homologous strain FA19. Strain MS11 is moderately serum resistant [49] and therefore may be more prone to bactericidal killing than strain FA19. It is interesting to note however that in our previous study, groups immunized with either full-length TbpA or TbpB chemically conjugated to Ctb had lower bactericidal antibody titers against strain MS11 than the groups immunized in this study with the chimeras [19]. The reason for this is uncertain, but one could speculate that antibodies generated against a greater variety of antigenic determinants may have resulted in the development of diversional, or blocking antibodies. These diversional antibodies may have negated the effects of bactericidal antibodies elicited against more sensitive domains. It could also be postulated that full-length Tbps generate the majority of their antibody repertoire to domains that are more antigenically variable; therefore antibodies elicited to such domains may be less cross-reactive against similar domains in heterologous strains. Interestingly, the antibodies from the animals immunized with the NB-Ctb chimera were not bactericidal against FA1090 at the lowest dilution tested; however, animals immunized with NB-L2-Ctb did generate bactericidal antibodies cross-reactive with this strain. Since the L2 epitope was the only difference between these chimeras, it is possible that antibodies elicited to L2 accounted for the bactericidal activity. It is also possible that improved killing was observed with the NB-L2 chimera because of greater stability of this chimera as discussed above. The relatively stable NB domain in the larger chimera may have resulted in presentation to the immune system of a more conserved and intact antigen compared to that presented by the NB-Ctb construct. Furthermore it is also a possibility that the L2 domain acted as a spacer to present more of the NB domain to the immune system. Although these issues cannot be ruled out, we have demonstrated previously, immunization with TbpA- and TbpB-Ctb conjugates together elicited antibodies that appeared to act in synergy, as this was the only serum capable of bactericidal killing against strain FA1090 [19]. In the present study, a similar synergistic effect may have resulted from immunization with the double chimera comprised of both NB and L2. Needless to say, more studies are necessary in order to discern whether L2 or other surface exposed loops of TbpA alone can elicit a bactericidal effect.
The question of whether generation of bactericidal antibodies affords immunity to gonococcal disease remains to be determined. Generation of opsonic antibodies could be critical. In addition, generation of genital tract IgA and/or IgG could prevent initial attachment of the gonococcus to the genital mucosa. In this study, we were unable to detect vaginal, TbpA-specific antibodies, but we were able to induce TbpB-specific antibodies of both IgA and IgG isotypes. We tested vaginal wash samples in vitro for antibodies that inhibited the growth of the gonococcus when provided transferrin as the sole iron source. Vaginal wash samples from animals immunized with either NB or NB-L2 inhibited the growth of the homologous strain, FA19. Only the vaginal wash samples from animals immunized with the NB-L2 chimeric antigens were capable of inhibiting the growth of the heterologous strain, FA1090. The ability to interfere with the growth of FA1090 was notable since the NB domains of FA19 and FA1090 only share 57% identity [13]. Although L2-specific vaginal antibodies were undetectable after five immunizations, we speculate that growth inhibition in the presence of double chimera-specific antibodies could be due to a higher degree of sequence identity between the L2 domains of FA19 and FA1090 (88%). At this point however, we do not know whether the presence of Tbp-specific antibodies in the growth medium inhibited growth due to iron deprivation or due to some other Tbp-dependent, but iron-acquisition independent mechanism.
In this study, we established that defined domains from the transferrin binding proteins could elicit a targeted immune response, specific for epitopes that may be sensitive to the bactericidal or growth-inhibitory effects of antibodies. This domain-specific approach could be useful in order to selectively avoid epitopes that are highly variable and could serve as immunogenic decoys. This study represents a proof-of-principle that chimeric vaccinogens comprised of Tbp and Ctb can induce relevant and potentially protective antibody responses. Future work in this laboratory will be aimed at enhancing the antibody response to L2 and other promising, well-conserved TbpA epitopes. Furthermore, because of the potential synergistic bactericidal effects of antibodies directed against TbpB and TbpA, other more conserved TbpB epitopes will be evaluated for their effectiveness in bactericidal killing and growth inhibition. Finally we intend to determine whether Tbp-specific antibodies can confer a protective phenotype in vivo using a murine gonococcal infection model [50].
Acknowledgements
Funding for this work was provided to C.N.C. by grant R01-AI047141 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health. Funding to M.W. R was provided by grant R01-DE06746 from the National Institute of Dental and Craniofacial Research, National Institutes of Health, and grant R01-AI46561 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health. G.A.P was supported by a Training in Molecular Pathogenesis grant (T32 AIO7617) from the NIH.
We gratefully acknowledge Christopher Thomas and P. Frederick Sparling for the contribution of anti-TbpB antiserum and Ann Jerse for critical reading of the manuscript. We also thank Heather R. Strange for excellent technican assistance with the animal studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tapsall J. Neisseria gonorrhoeae. World Health Organization; 2001. Antimicrobial resistance. [Google Scholar]
- 2.Gerbase AC, Rowley JT, Mertens TE. Global epidemiology of sexually transmitted diseases. Lancet. 1998;351 Supp 3:2–4. doi: 10.1016/s0140-6736(98)90001-0. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Sexually transmitted disease surveillance, 2004. Atlanta, GA: U.S. Department of Health and Human Services; 2004. [Google Scholar]
- 4.Russell MW, Sparling PF, Morrison RP, Cauci S, Fidel PL, Martin D, Hook EW, Mestecky J. Mucosal immunology of sexually transmitted diseases. 3rd ed. San Diego: Elsevier/Academic Press; 2005. pp. 1693–1720. [Google Scholar]
- 5.Hook EWI, Handsfield HH. Gonococcal infections in the adult. 3rd ed. New York: McGraw-Hill; 1999. pp. 451–466. [Google Scholar]
- 6.Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2003 supplement: gonococcal isolate surveillance project (GISP) annual report 2003. Atlanta, GA: U.S. Department of Health and Human Services; 2003. [Google Scholar]
- 7.Cohen MS, Hoffman IF, Royce RA, Kazembe P, Dyer JR, Daly CC, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV- 1. AIDSCAP Malawi Research Group. Lancet. 1997;349(9069):1868–1873. doi: 10.1016/s0140-6736(97)02190-9. [DOI] [PubMed] [Google Scholar]
- 8.McClelland RS, Wang CC, Mandaliya K, Overbaugh J, Reiner MT, Panteleeff DD, et al. Treatment of cervicitis is associated with decreased cervical shedding of HIV-1. Aids. 2001;15(1):105–110. doi: 10.1097/00002030-200101050-00015. [DOI] [PubMed] [Google Scholar]
- 9.Boslego JW, Tramont EC, Chung RC, McChesney DG, Ciak J, Sadoff JC, et al. Efficacy trial of a parenteral gonococcal pilus vaccine in men. Vaccine. 1991;9(3):154–162. doi: 10.1016/0264-410x(91)90147-x. [DOI] [PubMed] [Google Scholar]
- 10.Greenberg L, Diena BB, Ashton FA, Wallace R, Kenny CP, Znamirowski R, et al. Gonococcal vaccine studies in Inuvik. Can J Public Health. 1974;65(1):29–33. [PubMed] [Google Scholar]
- 11.Tramont EC. Gonococcal vaccines. Clin Microbiol Rev. 1989;(2 Suppl):S74–S77. doi: 10.1128/cmr.2.suppl.s74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornelissen CN, Anderson JE, Boulton IC, Sparling PF. Antigenic and sequence diversity in gonococcal transferrin-binding protein A. Infect Immun. 2000;68(8):4725–4735. doi: 10.1128/iai.68.8.4725-4735.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornelissen CN, Anderson JE, Sparling PF. Characterization of the diversity and the transferrin-binding domain of gonococcal transferrin-binding protein 2. Infect Immun. 1997;65(2):822–828. doi: 10.1128/iai.65.2.822-828.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lissolo L, Maitre-Wilmotte G, Dumas P, Mignon M, Danve D, Quentin-Millet M-J. Evaluation of transferrin-binding protein 2 within the transferrin-binding complex as a potential antigen for future meningococcal vaccines. Infect Immun. 1995;63(3):884–890. doi: 10.1128/iai.63.3.884-890.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pintor M, Ferron L, Gomez JA, Powell NB, Ala'Aldeen DA, Borriello SP, et al. Blocking of iron uptake from transferrin by antibodies against the transferring binding proteins in Neisseria meningitidis. Microb Pathog. 1996;20(3):127–139. doi: 10.1006/mpat.1996.0012. [DOI] [PubMed] [Google Scholar]
- 16.West D, Reddin K, Matheson M, Heath R, Funnell S, Hudson M, et al. Recombinant Neisseria meningitidis transferrin binding protein A protects against experimental meningococcal infection. Infect Immun. 2001;69(3):1561–1567. doi: 10.1128/IAI.69.3.1561-1567.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevenson P, Williams P, Griffiths E. Common antigemic domains in transferring-binding protein 2 of Neisseria meningitidis, Neisseria gonorrhoeae, and Haemophilus influenzae Type b. Infect Immun. 1992;60(6):2391–2396. doi: 10.1128/iai.60.6.2391-2396.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cornelissen CN, Kelley M, Hobbs MM, Anderson JE, Cannon JG, Cohen MS, et al. The transferrin receptor expressed by gonococcal strain FA1090 is required for the experimental infection of human male volunteers. Mol Microbiol. 1998;27(3):611–616. doi: 10.1046/j.1365-2958.1998.00710.x. [DOI] [PubMed] [Google Scholar]
- 19.Price GA, Russell MW, Cornelissen CN. Intranasal administration of recombinant Neisseria gonorrhoeae transferrin binding proteins A and B conjugated to the cholera toxin B subunit induces systemic and vaginal antibodies in mice. Infect Immun. 2005;73(7):3945–3953. doi: 10.1128/IAI.73.7.3945-3953.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hajishengallis G, Arce S, Gockel CM, Connell TD, Russell MW. Immunomodulation with enterotoxins for the generation of secretory immunity or tolerance: applications for oral infections. J Dent Res. 2005;84(12):1104–1116. doi: 10.1177/154405910508401205. [DOI] [PubMed] [Google Scholar]
- 21.De Haan L, Hirst TR. Cholera toxin: a paradigm for multi-functional engagement of cellular mechanisms (Review) Mol Membr Biol. 2004;21(2):77–92. doi: 10.1080/09687680410001663267. [DOI] [PubMed] [Google Scholar]
- 22.Hardy SJ, Holmgren J, Johansson S, Sanchez J, Hirst TR. Coordinated assembly of multisubunit proteins: oligomerization of bacterial enterotoxins in vivo and in vitro. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(19):7109–7113. doi: 10.1073/pnas.85.19.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hajishengallis G, Hollingshead SK, Koga T, Russell MW. Mucosal immunization with a bacterial protein antigen genetically coupled to cholera toxin A2/B subunits. J Immunol. 1995;154(9):4322–4332. [PubMed] [Google Scholar]
- 24.Lee SF, Halperin SA, Salloum DF, MacMillan A, Morris A. Mucosal immunization with a genetically engineered pertussis toxin S1 fragment-cholera toxin subunit B chimeric protein. Infect Immun. 2003;71(4):2272–2275. doi: 10.1128/IAI.71.4.2272-2275.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Erbe JL, Lockatell CV, Johnson DE, Jobling MG, Holmes RK, et al. Use of translational fusion of the MrpH fimbrial adhesin-binding domain with the cholera toxin A2 domain, coexpressed with the cholera toxin B subunit, as an intranasal vaccine to prevent experimental urinary tract infection by Proteus mirabilis. Infect Immun. 2004;72(12):7306–7310. doi: 10.1128/IAI.72.12.7306-7310.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin M, Hajishengallis G, Metzger DJ, Michalek SM, Connell TD, Russell MW. Recombinant antigen-enterotoxin A2/B chimeric mucosal immunogens differentially enhance antibody responses and B7-dependent costimulation of CD4(+) T cells. Infect Immun. 2001;69(1):252–261. doi: 10.1128/IAI.69.1.252-261.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheoran AS, Artiushin S, Timoney JF. Nasal mucosal immunogenicity for the horse of a SeM peptide of Streptococcus equi genetically coupled to cholera toxin. Vaccine. 2002 Feb 22;20(11–12):1653–1659. doi: 10.1016/s0264-410x(01)00488-1. [DOI] [PubMed] [Google Scholar]
- 28.Sultan F, Jin LL, Jobling MG, Holmes RK, Stanley SL., Jr Mucosal immunogenicity of a holotoxin-like molecule containing the serine-rich Entamoeba histolytica protein (SREHP) fused to the A2 domain of cholera toxin. Infect Immun. 1998 Feb;66(2):462–468. doi: 10.1128/iai.66.2.462-468.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh SR, Hulett K, Pillai SR, Dennis VA, Oh MK, Scissum-Gunn K. Mucosal immunization with recombinant MOMP genetically linked with modified cholera toxin confers protection against Chlamydia trachomatis infection. Vaccine. 2006 Feb 20;24(8):1213–1224. doi: 10.1016/j.vaccine.2005.08.097. [DOI] [PubMed] [Google Scholar]
- 30.Kellogg DS, Jr, Peacock WL, Jr, Deacon WE, Brown L, Pirkle CI. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J Bacteriol. 1963;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.West SEH, Sparling PF. Aerobactin utilization by Neisseria gonorrhoeae and cloning of a genomic DNA fragment that complements Escherichia coli fhuB mutations. J Bacteriol. 1987;169(8):3414–3421. doi: 10.1128/jb.169.8.3414-3421.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masri HP, Cornelissen CN. Specific ligand binding attributable to individual epitopes of gonococcal transferrin binding protein A. Infect Immun. 2002;70(2):732–740. doi: 10.1128/iai.70.2.732-740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Price GA, Hobbs MM, Cornelissen CN. Immunogenicity of gonococcal transferrin binding proteins during natural infections. Infect Immun. 2004 January 1;72(1):277–283. doi: 10.1128/IAI.72.1.277-283.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenthal KL, Gallichan WS. Challenges for vaccination against sexually-transmitted diseases: induction and long-term maintenance of mucosal immune responses in the female genital tract. Semin Immunol. 1997;9(5):303–314. doi: 10.1006/smim.1997.0086. [DOI] [PubMed] [Google Scholar]
- 35.Yost-Daljev MK, Cornelissen CN. Determination of surface-exposed, functional domains of gonococcal transferrin-binding protein A. Infect Immun. 2004;72(3):1775–1785. doi: 10.1128/IAI.72.3.1775-1785.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snyder LA, Davies JK, Saunders NJ. Microarray genomotyping of key experimental strains of Neisseria gonorrhoeae reveals gene complement diversity and five new neisserial genes associated with Minimal Mobile Elements. BMC Genomics. 2004;5(1):23. doi: 10.1186/1471-2164-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jordan PW, Snyder LA, Saunders NJ. Strain-specific differences in Neisseria gonorrhoeae associated with the phase variable gene repertoire. BMC Microbiology. 2005;5(1):21. doi: 10.1186/1471-2180-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jobling MG, Palmer LM, Erbe JL, Holmes RK. Construction and characterization of versatile cloning vectors for efficient delivery of native foreign proteins to the periplasm of Escherichia coli. Plasmid. 1997;38(3):158–173. doi: 10.1006/plas.1997.1309. [DOI] [PubMed] [Google Scholar]
- 39.Retzer MD, Yu RH, Schryvers AB. Identification of sequences in human transferrin that bind to the bacterial receptor protein, transferrin-binding protein B. Mol Microbiol. 1999;32(1):111–121. doi: 10.1046/j.1365-2958.1999.01331.x. [DOI] [PubMed] [Google Scholar]
- 40.Matoba N, Magerus A, Geyer BC, Zhang Y, Muralidharan M, Alfsen A, et al. A mucosally targeted subunit vaccine candidate eliciting HIV-1 transcytosis-blocking Abs. Proc Natl Acad Sci U S A. 2004;101(37):13584–13589. doi: 10.1073/pnas.0405297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russell MW, Mestecky J. Humoral immune responses to microbial infections in the genital tract. Microbes Infect. 2002;4(6):667–677. doi: 10.1016/s1286-4579(02)01585-x. [DOI] [PubMed] [Google Scholar]
- 42.Anderson JE, Sparling PF, Cornelissen CN. Gonococcal transferrin-binding protein 2 facilitates but is not essential for transferrin utilization. J Bacteriol. 1994;176(11):3162–3170. doi: 10.1128/jb.176.11.3162-3170.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Legrain M, Mazarin V, Irwin SE, Bouchon B, Quentin-Millet MJ, Jacobs E, et al. Cloning and characterization of Neisseria meningitidis genes encoding the transferrin-binding proteins Tbp1 and Tbp2. Gene. 1993;130(1):73–80. doi: 10.1016/0378-1119(93)90348-7. [DOI] [PubMed] [Google Scholar]
- 44.Norheim G, Aase A, Caugant DA, Hoiby EA, Fritzsonn E, Tangen T, et al. Development and characterisation of outer membrane vesicle vaccines against serogroup A Neisseria meningitidis. Vaccine. 2005;23(29):3762–3774. doi: 10.1016/j.vaccine.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 45.Boulton IC, Yost MK, Anderson JE, Cornelissen CN. Identification of discrete domains within gonococcal transferrin- binding protein A that are necessary for ligand binding and iron uptake functions. Infect Immun. 2000;68(12):6988–6996. doi: 10.1128/iai.68.12.6988-6996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oakhill JS, Sutton BJ, Gorringe AR, Evans RW. Homology modelling of transferrin-binding protein A from Neisseria meningitidis. Protein Engineering, Design & Selection. 2005;18(5):221–228. doi: 10.1093/protein/gzi024. [DOI] [PubMed] [Google Scholar]
- 47.Gorringe AR, Borrow R, Fox AJ, Robinson A. Human antibody response to meningococcal transferrin binding proteins: evidence for vaccine potential. Vaccine. 1995;13(13):1207–1212. doi: 10.1016/0264-410x(95)00055-6. [DOI] [PubMed] [Google Scholar]
- 48.Sadler K, Zeng W, Jackosn DC. Synthetic peptide epitope-based polymers: controlling size and determining the efficiency of epitope incorporation. J Pept Res. 2002;60(3):150–158. doi: 10.1034/j.1399-3011.2002.21009.x. [DOI] [PubMed] [Google Scholar]
- 49.Carbonetti N, Simnad V, Elkins C, Sparling PF. Construction of isogenic gonococci with variable porin structure: effects on susceptibility to human serum and antibiotics. Mol Microbiol. 1990;4(6):1009–1018. doi: 10.1111/j.1365-2958.1990.tb00673.x. [DOI] [PubMed] [Google Scholar]
- 50.Jerse AE. Experimental gonococcal genital tract infection and opacity protein expression in estradiol-treated mice. Infect Immun. 1999;67(11):5699–5708. doi: 10.1128/iai.67.11.5699-5708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maniatis T, Fritsch EF, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 52.Kenney CD, Cornelissen CN. Demonstration and characterization of a specific interaction between gonococcal transferrin binding protein A and TonB. J Bacteriol. 2002;184(22):6138–6145. doi: 10.1128/JB.184.22.6138-6145.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mickelsen PA, Sparling PF. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from transferrin and iron compounds. Infect Immun. 1981;33(2):555–564. doi: 10.1128/iai.33.2.555-564.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meyer TF, Mlawer N, So M. Pilus expression in Neisseria gonorrhoeae involves chromosomal rearrangement. Cell. 1982;30(1):45–52. doi: 10.1016/0092-8674(82)90010-1. [DOI] [PubMed] [Google Scholar]
- 55.Cohen MS, Cannon JG, Jerse AE, Charniga LM, Isbey SF, Whicker LG. Human experimentation with Neisseria gonorrhoeae: Rationale, methods, and implications for the biology of infection and vaccine development. J Infect Dis. 1994;169:532–537. doi: 10.1093/infdis/169.3.532. [DOI] [PubMed] [Google Scholar]


