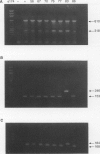
Abstract
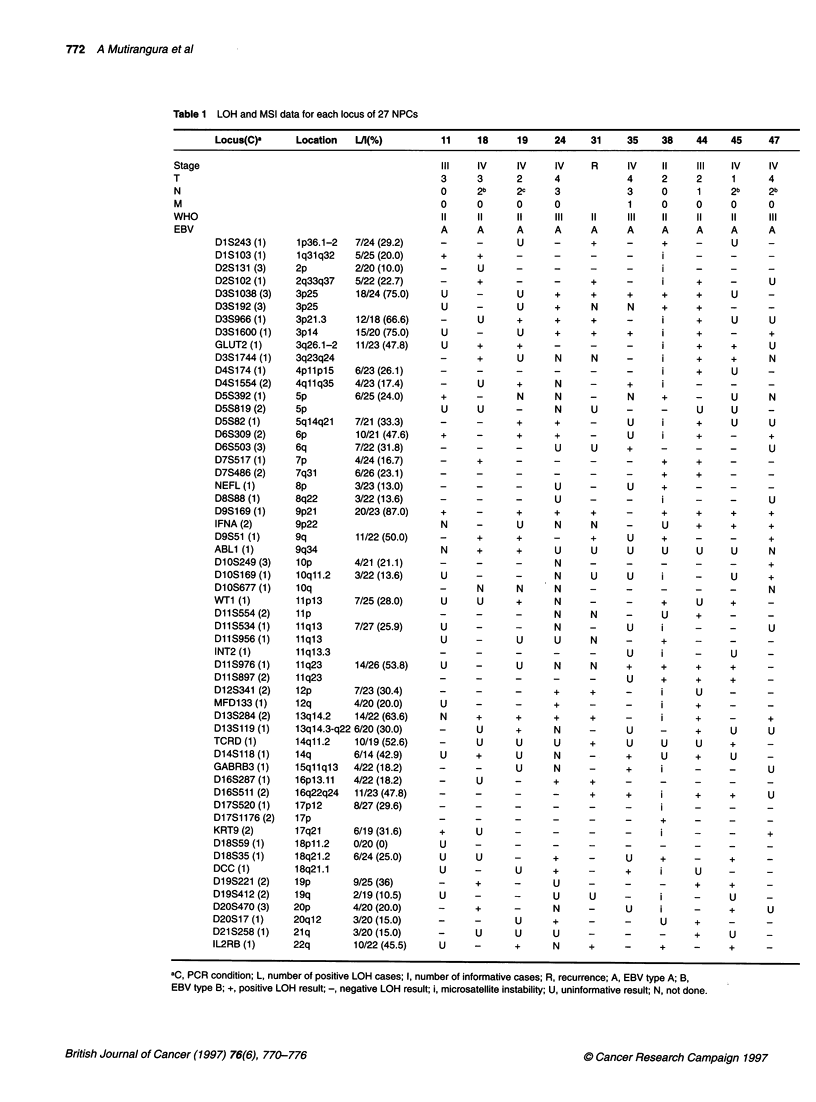
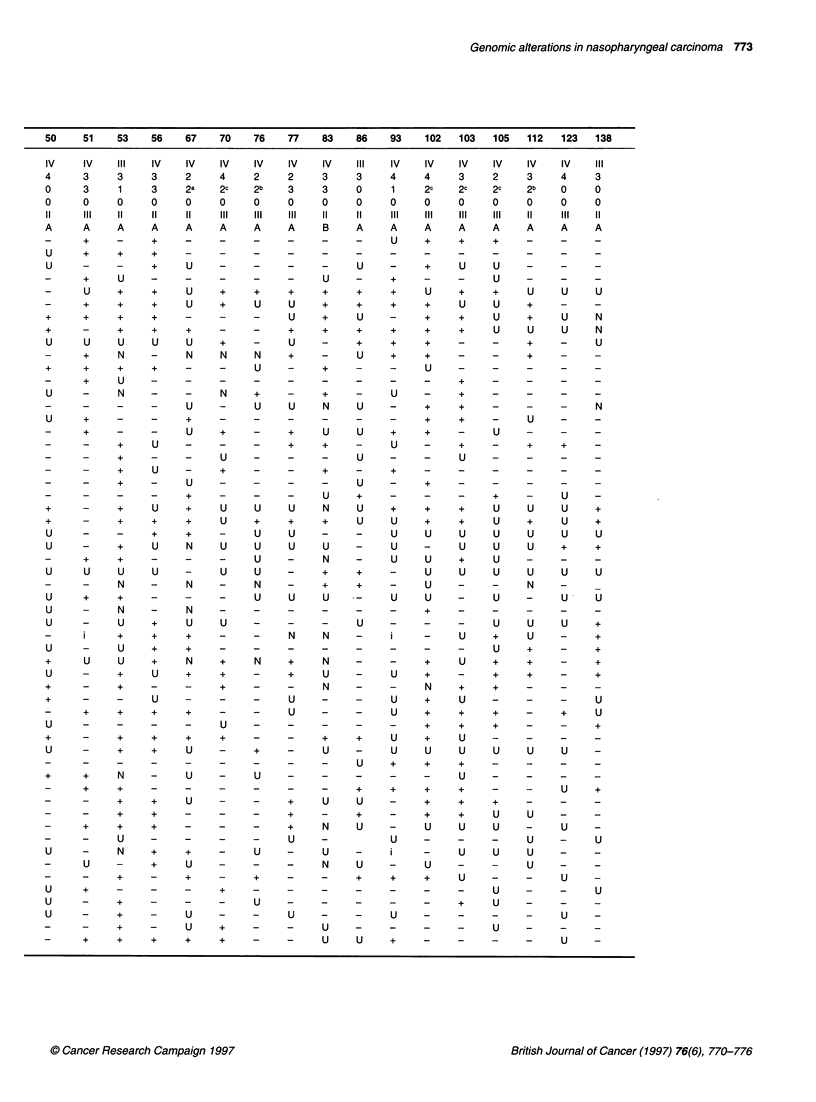
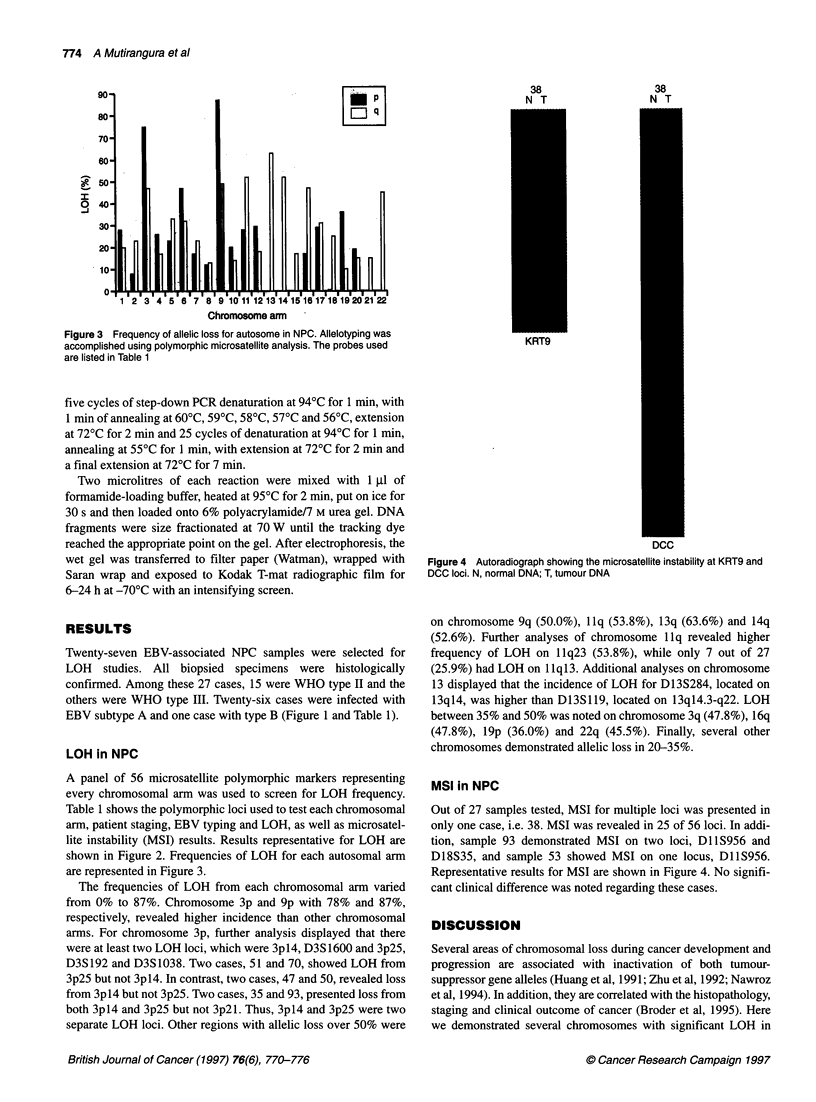
Nasopharyngeal carcinoma is a subset of head and neck squamous cell cancers with unique endemic distribution and aetiological co-factors. Epstein-Barr virus has been revealed to be an important aetiological factor for most nasopharyngeal carcinomas. Nevertheless, additional genetic alterations may be involved in their development and progression. The aim of this study was to determine the likely chromosomal locations of tumour-suppressor genes related to Epstein-Barr virus-associated nasopharyngeal carcinoma. Fifty-six microsatellite polymorphic markers located on every autosomal arm were used to estimate the incidence of loss of heterozygosity in 27 Epstein-Barr virus-associated nasopharyngeal carcinomas. High frequencies of allelic loss were observed on chromosome 3p (75.0%) and 9p (87.0%). Chromosome 9q, 11q, 13q and 14q displayed loss in over 50%, while chromosome 3q, 6p, 16q, 19q and 22q exhibited loss in 35-50%. Furthermore, several other chromosomal arms demonstrated allelic loss in 20-35%. Additionally, 1 of the 27 cases showed microsatellite instability at multiple loci. These findings provide evidence of multiple genetic alterations during cancer development and clues for further studies of tumour-suppressor genes in Epstein-Barr virus-associated nasopharyngeal carcinoma.
Full text
PDF
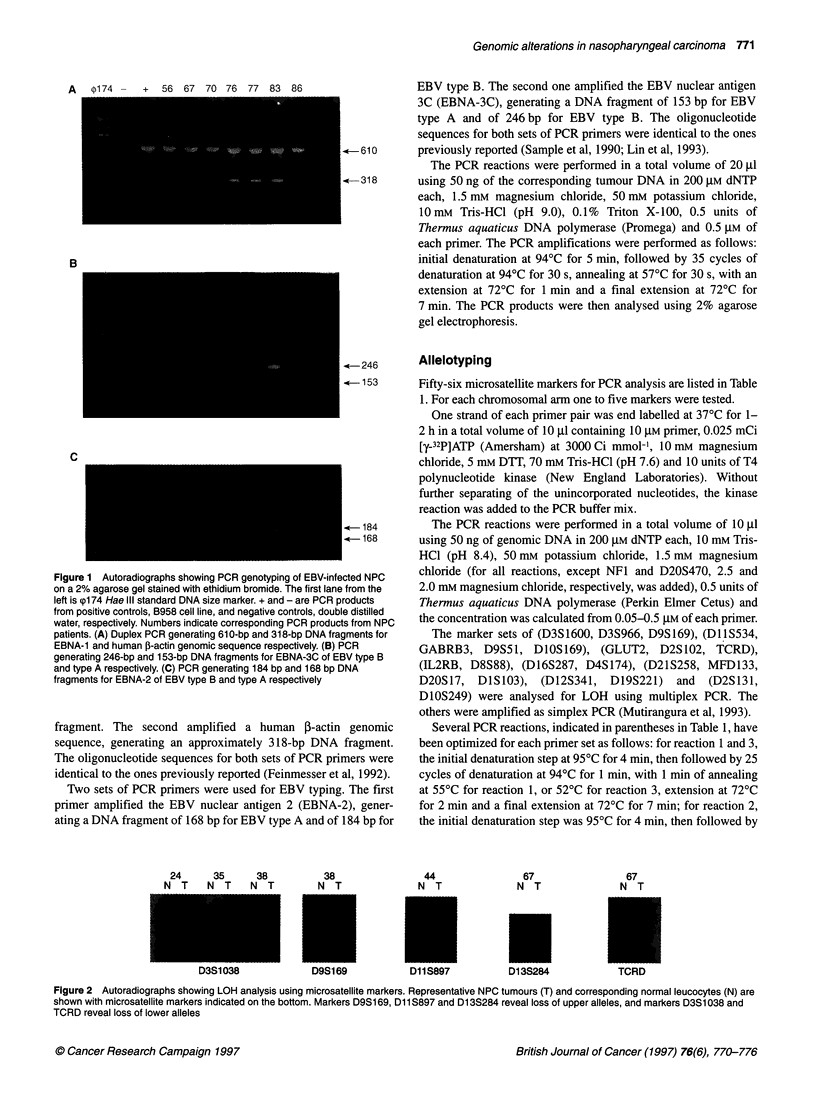


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyle J. O., Hakim J., Koch W., van der Riet P., Hruban R. H., Roa R. A., Correo R., Eby Y. J., Ruppert J. M., Sidransky D. The incidence of p53 mutations increases with progression of head and neck cancer. Cancer Res. 1993 Oct 1;53(19):4477–4480. [PubMed] [Google Scholar]
- Brennan J. A., Mao L., Hruban R. H., Boyle J. O., Eby Y. J., Koch W. M., Goodman S. N., Sidransky D. Molecular assessment of histopathological staging in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995 Feb 16;332(7):429–435. doi: 10.1056/NEJM199502163320704. [DOI] [PubMed] [Google Scholar]
- Broder S., Karp J. E. Progress against cancer. J Cancer Res Clin Oncol. 1995;121(11):633–647. doi: 10.1007/BF01218521. [DOI] [PubMed] [Google Scholar]
- Carson D. A., Lois A. Cancer progression and p53. Lancet. 1995 Oct 14;346(8981):1009–1011. doi: 10.1016/s0140-6736(95)91693-8. [DOI] [PubMed] [Google Scholar]
- Chakrani F., Armand J. P., Lenoir G., Ju L. Y., Liang J. P., May E., May P. Mutations clustered in exon 5 of the p53 gene in primary nasopharyngeal carcinomas from southeastern Asia. Int J Cancer. 1995 May 4;61(3):316–320. doi: 10.1002/ijc.2910610307. [DOI] [PubMed] [Google Scholar]
- Chang W. Y., Cairns P., Schoenberg M. P., Polascik T. J., Sidransky D. Novel suppressor loci on chromosome 14q in primary bladder cancer. Cancer Res. 1995 Aug 1;55(15):3246–3249. [PubMed] [Google Scholar]
- Choi P. H., Suen M. W., Huang D. P., Lo K. W., Lee J. C. Nasopharyngeal carcinoma: genetic changes, Epstein-Barr virus infection, or both. A clinical and molecular study of 36 patients. Cancer. 1993 Nov 15;72(10):2873–2878. doi: 10.1002/1097-0142(19931115)72:10<2873::aid-cncr2820721003>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Fandi A., Altun M., Azli N., Armand J. P., Cvitkovic E. Nasopharyngeal cancer: epidemiology, staging, and treatment. Semin Oncol. 1994 Jun;21(3):382–397. [PubMed] [Google Scholar]
- Feinmesser R., Miyazaki I., Cheung R., Freeman J. L., Noyek A. M., Dosch H. M. Diagnosis of nasopharyngeal carcinoma by DNA amplification of tissue obtained by fine-needle aspiration. N Engl J Med. 1992 Jan 2;326(1):17–21. doi: 10.1056/NEJM199201023260103. [DOI] [PubMed] [Google Scholar]
- Field J. K., Kiaris H., Risk J. M., Tsiriyotis C., Adamson R., Zoumpourlis V., Rowley H., Taylor K., Whittaker J., Howard P. Allelotype of squamous cell carcinoma of the head and neck: fractional allele loss correlates with survival. Br J Cancer. 1995 Nov;72(5):1180–1188. doi: 10.1038/bjc.1995.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field J. K., Spandidos D. A., Malliri A., Gosney J. R., Yiagnisis M., Stell P. M. Elevated P53 expression correlates with a history of heavy smoking in squamous cell carcinoma of the head and neck. Br J Cancer. 1991 Sep;64(3):573–577. doi: 10.1038/bjc.1991.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong C. T., White P. S., Peterson K., Sapienza C., Cavenee W. K., Kern S. E., Vogelstein B., Cantor A. B., Look A. T., Brodeur G. M. Loss of heterozygosity for chromosomes 1 or 14 defines subsets of advanced neuroblastomas. Cancer Res. 1992 Apr 1;52(7):1780–1785. [PubMed] [Google Scholar]
- Gudmundsson J., Barkardottir R. B., Eiriksdottir G., Baldursson T., Arason A., Egilsson V., Ingvarsson S. Loss of heterozygosity at chromosome 11 in breast cancer: association of prognostic factors with genetic alterations. Br J Cancer. 1995 Sep;72(3):696–701. doi: 10.1038/bjc.1995.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudmundsson J., Johannesdottir G., Bergthorsson J. T., Arason A., Ingvarsson S., Egilsson V., Barkardottir R. B. Different tumor types from BRCA2 carriers show wild-type chromosome deletions on 13q12-q13. Cancer Res. 1995 Nov 1;55(21):4830–4832. [PubMed] [Google Scholar]
- Huang D. P., Lo K. W., Choi P. H., Ng A. Y., Tsao S. Y., Yiu G. K., Lee J. C. Loss of heterozygosity on the short arm of chromosome 3 in nasopharyngeal carcinoma. Cancer Genet Cytogenet. 1991 Jul 1;54(1):91–99. doi: 10.1016/0165-4608(91)90035-s. [DOI] [PubMed] [Google Scholar]
- Huang D. P., Lo K. W., van Hasselt C. A., Woo J. K., Choi P. H., Leung S. F., Cheung S. T., Cairns P., Sidransky D., Lee J. C. A region of homozygous deletion on chromosome 9p21-22 in primary nasopharyngeal carcinoma. Cancer Res. 1994 Aug 1;54(15):4003–4006. [PubMed] [Google Scholar]
- Hui A. B., Lo K. W., Leung S. F., Choi P. H., Fong Y., Lee J. C., Huang D. P. Loss of heterozygosity on the long arm of chromosome 11 in nasopharyngeal carcinoma. Cancer Res. 1996 Jul 15;56(14):3225–3229. [PubMed] [Google Scholar]
- Iizuka M., Sugiyama Y., Shiraishi M., Jones C., Sekiya T. Allelic losses in human chromosome 11 in lung cancers. Genes Chromosomes Cancer. 1995 May;13(1):40–46. doi: 10.1002/gcc.2870130107. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971 Apr;68(4):820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latif F., Tory K., Gnarra J., Yao M., Duh F. M., Orcutt M. L., Stackhouse T., Kuzmin I., Modi W., Geil L. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993 May 28;260(5112):1317–1320. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- Liebowitz D. Nasopharyngeal carcinoma: the Epstein-Barr virus association. Semin Oncol. 1994 Jun;21(3):376–381. [PubMed] [Google Scholar]
- Lin J. C., Lin S. C., De B. K., Chan W. P., Evatt B. L., Chan W. C. Precision of genotyping of Epstein-Barr virus by polymerase chain reaction using three gene loci (EBNA-2, EBNA-3C, and EBER): predominance of type A virus associated with Hodgkin's disease. Blood. 1993 Jun 15;81(12):3372–3381. [PubMed] [Google Scholar]
- Lo K. W., Cheung S. T., Leung S. F., van Hasselt A., Tsang Y. S., Mak K. F., Chung Y. F., Woo J. K., Lee J. C., Huang D. P. Hypermethylation of the p16 gene in nasopharyngeal carcinoma. Cancer Res. 1996 Jun 15;56(12):2721–2725. [PubMed] [Google Scholar]
- Lo K. W., Huang D. P., Lau K. M. p16 gene alterations in nasopharyngeal carcinoma. Cancer Res. 1995 May 15;55(10):2039–2043. [PubMed] [Google Scholar]
- Maestro R., Gasparotto D., Vukosavljevic T., Barzan L., Sulfaro S., Boiocchi M. Three discrete regions of deletion at 3p in head and neck cancers. Cancer Res. 1993 Dec 1;53(23):5775–5779. [PubMed] [Google Scholar]
- Meredith S. D., Levine P. A., Burns J. A., Gaffey M. J., Boyd J. C., Weiss L. M., Erickson N. L., Williams M. E. Chromosome 11q13 amplification in head and neck squamous cell carcinoma. Association with poor prognosis. Arch Otolaryngol Head Neck Surg. 1995 Jul;121(7):790–794. doi: 10.1001/archotol.1995.01890070076016. [DOI] [PubMed] [Google Scholar]
- Mutirangura A., Greenberg F., Butler M. G., Malcolm S., Nicholls R. D., Chakravarti A., Ledbetter D. H. Multiplex PCR of three dinucleotide repeats in the Prader-Willi/Angelman critical region (15q11-q13): molecular diagnosis and mechanism of uniparental disomy. Hum Mol Genet. 1993 Feb;2(2):143–151. doi: 10.1093/hmg/2.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawroz H., van der Riet P., Hruban R. H., Koch W., Ruppert J. M., Sidransky D. Allelotype of head and neck squamous cell carcinoma. Cancer Res. 1994 Mar 1;54(5):1152–1155. [PubMed] [Google Scholar]
- Rhyu M. S. Molecular mechanisms underlying hereditary nonpolyposis colorectal carcinoma. J Natl Cancer Inst. 1996 Mar 6;88(5):240–251. doi: 10.1093/jnci/88.5.240. [DOI] [PubMed] [Google Scholar]
- Sample J., Young L., Martin B., Chatman T., Kieff E., Rickinson A., Kieff E. Epstein-Barr virus types 1 and 2 differ in their EBNA-3A, EBNA-3B, and EBNA-3C genes. J Virol. 1990 Sep;64(9):4084–4092. doi: 10.1128/jvi.64.9.4084-4092.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitsky K., Bar-Shira A., Gilad S., Rotman G., Ziv Y., Vanagaite L., Tagle D. A., Smith S., Uziel T., Sfez S. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science. 1995 Jun 23;268(5218):1749–1753. doi: 10.1126/science.7792600. [DOI] [PubMed] [Google Scholar]
- Shin D. M., Kim J., Ro J. Y., Hittelman J., Roth J. A., Hong W. K., Hittelman W. N. Activation of p53 gene expression in premalignant lesions during head and neck tumorigenesis. Cancer Res. 1994 Jan 15;54(2):321–326. [PubMed] [Google Scholar]
- Sun Y., Hegamyer G., Colburn N. H. Nasopharyngeal carcinoma shows no detectable retinoblastoma susceptibility gene alterations. Oncogene. 1993 Mar;8(3):791–795. [PubMed] [Google Scholar]
- Thibodeau S. N., Bren G., Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993 May 7;260(5109):816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- Young J., Leggett B., Ward M., Thomas L., Buttenshaw R., Searle J., Chenevix-Trench G. Frequent loss of heterozygosity on chromosome 14 occurs in advanced colorectal carcinomas. Oncogene. 1993 Mar;8(3):671–675. [PubMed] [Google Scholar]
- Zhu X., Dunn J. M., Goddard A. D., Squire J. A., Becker A., Phillips R. A., Gallie B. L. Mechanisms of loss of heterozygosity in retinoblastoma. Cytogenet Cell Genet. 1992;59(4):248–252. doi: 10.1159/000133261. [DOI] [PubMed] [Google Scholar]
- el-Naggar A. K., Hurr K., Batsakis J. G., Luna M. A., Goepfert H., Huff V. Sequential loss of heterozygosity at microsatellite motifs in preinvasive and invasive head and neck squamous carcinoma. Cancer Res. 1995 Jun 15;55(12):2656–2659. [PubMed] [Google Scholar]