Abstract
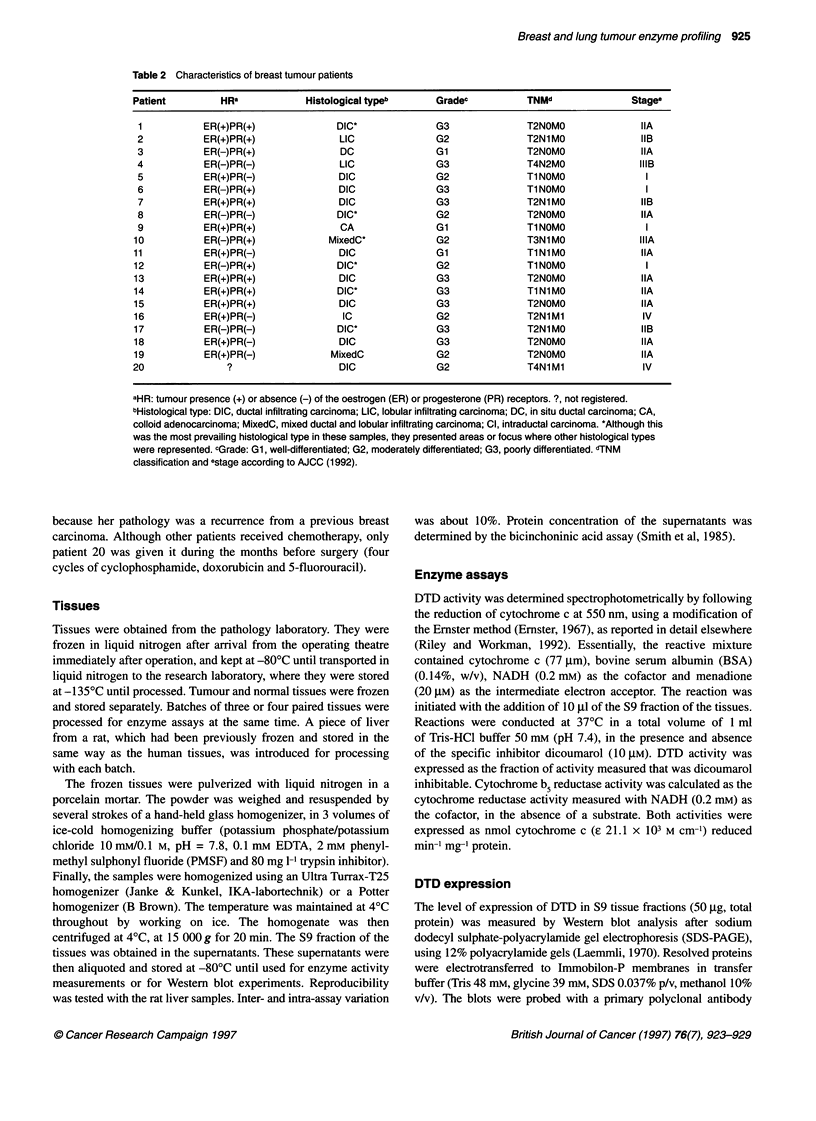
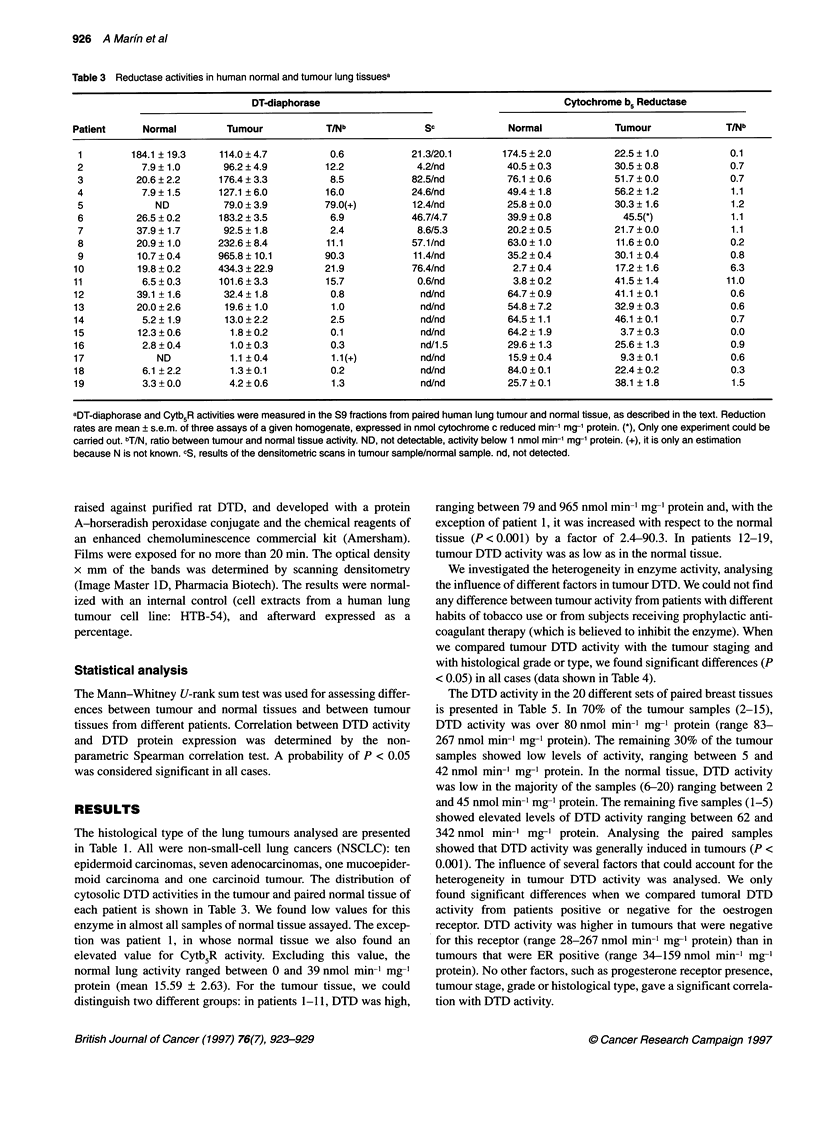
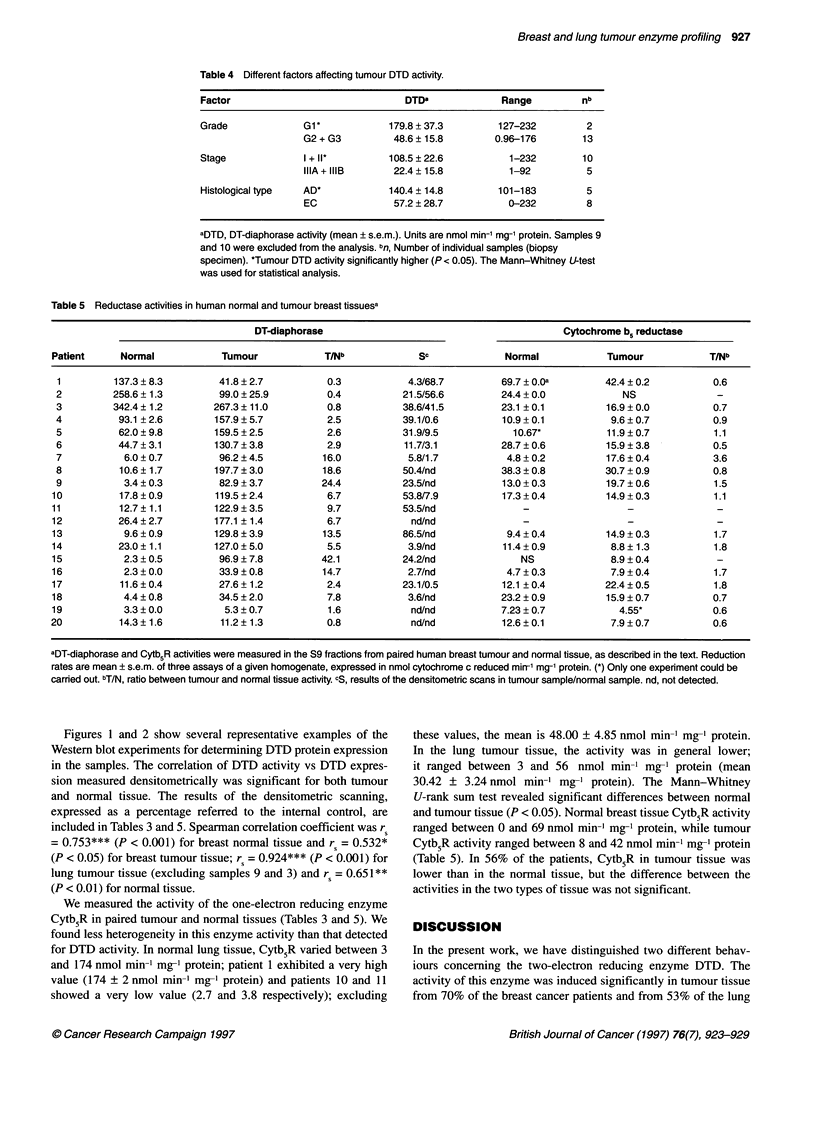
The level of expression of enzymes that can activate or detoxify bioreductive agents within tumours has emerged as an important feature in the development of these anti-tumour compounds. The levels of two such reductase enzymes have been determined in 19 human non-small-cell lung tumours and 20 human breast tumours, together with the corresponding normal tissue. DT-diaphorase (DTD) enzyme levels (both expression and activity) were determined in these samples. Cytochrome b5 reductase (Cytb5R) activity was also assessed. With the exception of six patients, the levels of DTD activity were below 45 nmol min(-1) mg(-1) in the normal tissues assayed. DTD tumour activity was extremely variable, distinguishing two different groups of patients, one with DTD activity above 79 nmol min(-1) mg(-1) and the other with levels that were in the same range as found for the normal tissues. In 53% of the lung tumour samples, DTD activity was increased with respect to the normal tissue by a factor of 2.4-90.3 (range 79-965 nmol min[-1] mg[-1]). In 70% of the breast tumour samples, DTD activity was over 80 nmol min(-1) mg(-1) (range 83-267 nmol min[-1] mg[-1]). DTD expression measured by Western blot correlated well with the enzyme activity measured in both tumour and normal tissues. The levels of the other reductase enzyme, Cytb5R, were not as variable as those for DTD, being in the same range in both tumour and normal tissue or slightly higher in the normal tissues. The heterogeneous nature of DTD activity and expression reinforces the need to measure enzyme levels in individual patients before therapy with DTD-activated bioreductive drugs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belinsky M., Jaiswal A. K. NAD(P)H:quinone oxidoreductase1 (DT-diaphorase) expression in normal and tumor tissues. Cancer Metastasis Rev. 1993 Jun;12(2):103–117. doi: 10.1007/BF00689804. [DOI] [PubMed] [Google Scholar]
- Chesis P. L., Levin D. E., Smith M. T., Ernster L., Ames B. N. Mutagenicity of quinones: pathways of metabolic activation and detoxification. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1696–1700. doi: 10.1073/pnas.81.6.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresteil T., Jaiswal A. K. High levels of expression of the NAD(P)H:quinone oxidoreductase (NQO1) gene in tumor cells compared to normal cells of the same origin. Biochem Pharmacol. 1991 Aug 8;42(5):1021–1027. doi: 10.1016/0006-2952(91)90284-c. [DOI] [PubMed] [Google Scholar]
- Doherty N., Hancock S. L., Kaye S., Coleman C. N., Shulman L., Marquez C., Mariscal C., Rampling R., Senan S., Roemeling R. V. Muscle cramping in phase I clinical trials of tirapazamine (SR 4233) with and without radiation. Int J Radiat Oncol Biol Phys. 1994 May 15;29(2):379–382. doi: 10.1016/0360-3016(94)90293-3. [DOI] [PubMed] [Google Scholar]
- Edwards Y. H., Potter J., Hopkinson D. A. Human FAD-dependent NAD(P)H diaphorase. Biochem J. 1980 May 1;187(2):429–436. doi: 10.1042/bj1870429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimmons S. A., Workman P., Grever M., Paull K., Camalier R., Lewis A. D. Reductase enzyme expression across the National Cancer Institute Tumor cell line panel: correlation with sensitivity to mitomycin C and EO9. J Natl Cancer Inst. 1996 Mar 6;88(5):259–269. doi: 10.1093/jnci/88.5.259. [DOI] [PubMed] [Google Scholar]
- Hodnick W. F., Sartorelli A. C. Reductive activation of mitomycin C by NADH:cytochrome b5 reductase. Cancer Res. 1993 Oct 15;53(20):4907–4912. [PubMed] [Google Scholar]
- Iyanagi T., Yamazaki I. One-electron-transfer reactions in biochemical systems. 3. One-electron reduction of quinones by microsomal flavin enzymes. Biochim Biophys Acta. 1969 Apr 8;172(3):370–381. doi: 10.1016/0005-2728(69)90133-9. [DOI] [PubMed] [Google Scholar]
- Jaiswal A. K., Burnett P., Adesnik M., McBride O. W. Nucleotide and deduced amino acid sequence of a human cDNA (NQO2) corresponding to a second member of the NAD(P)H:quinone oxidoreductase gene family. Extensive polymorphism at the NQO2 gene locus on chromosome 6. Biochemistry. 1990 Feb 20;29(7):1899–1906. doi: 10.1021/bi00459a034. [DOI] [PubMed] [Google Scholar]
- Kennedy K. A., Teicher B. A., Rockwell S., Sartorelli A. C. The hypoxic tumor cell: a target for selective cancer chemotherapy. Biochem Pharmacol. 1980 Jan 1;29(1):1–8. doi: 10.1016/0006-2952(80)90235-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lind C. Relationship between the rate of reduction of benzo(a)pyrene-3,6-quinone and the formation of benzo(a)pyrene-3,6-quinol glucuronides in rat liver microsomes. Biochem Pharmacol. 1985 Mar 15;34(6):895–897. doi: 10.1016/0006-2952(85)90772-5. [DOI] [PubMed] [Google Scholar]
- Plumb J. A., Gerritsen M., Milroy R., Thomson P., Workman P. Relative importance of DT-diaphorase and hypoxia in the bioactivation of EO9 by human lung tumor cell lines. Int J Radiat Oncol Biol Phys. 1994 May 15;29(2):295–299. doi: 10.1016/0360-3016(94)90278-x. [DOI] [PubMed] [Google Scholar]
- Plumb J. A., Gerritsen M., Workman P. DT-diaphorase protects cells from the hypoxic cytotoxicity of indoloquinone EO9. Br J Cancer. 1994 Dec;70(6):1136–1143. doi: 10.1038/bjc.1994.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska H. J., Talalay P. Regulatory mechanisms of monofunctional and bifunctional anticarcinogenic enzyme inducers in murine liver. Cancer Res. 1988 Sep 1;48(17):4776–4782. [PubMed] [Google Scholar]
- Prochaska H. J., Talalay P., Sies H. Direct protective effect of NAD(P)H:quinone reductase against menadione-induced chemiluminescence of postmitochondrial fractions of mouse liver. J Biol Chem. 1987 Feb 15;262(5):1931–1934. [PubMed] [Google Scholar]
- Riley R. J., Workman P. DT-diaphorase and cancer chemotherapy. Biochem Pharmacol. 1992 Apr 15;43(8):1657–1669. doi: 10.1016/0006-2952(92)90694-e. [DOI] [PubMed] [Google Scholar]
- Robertson N., Haigh A., Adams G. E., Stratford I. J. Factors affecting sensitivity to EO9 in rodent and human tumour cells in vitro: DT-diaphorase activity and hypoxia. Eur J Cancer. 1994;30A(7):1013–1019. doi: 10.1016/0959-8049(94)90134-1. [DOI] [PubMed] [Google Scholar]
- Sartorelli A. C. Therapeutic attack of hypoxic cells of solid tumors: presidential address. Cancer Res. 1988 Feb 15;48(4):775–778. [PubMed] [Google Scholar]
- Schellens J. H., Planting A. S., van Acker B. A., Loos W. J., de Boer-Dennert M., van der Burg M. E., Koier I., Krediet R. T., Stoter G., Verweij J. Phase I and pharmacologic study of the novel indoloquinone bioreductive alkylating cytotoxic drug E09. J Natl Cancer Inst. 1994 Jun 15;86(12):906–912. doi: 10.1093/jnci/86.12.906. [DOI] [PubMed] [Google Scholar]
- Schlager J. J., Powis G. Cytosolic NAD(P)H:(quinone-acceptor)oxidoreductase in human normal and tumor tissue: effects of cigarette smoking and alcohol. Int J Cancer. 1990 Mar 15;45(3):403–409. doi: 10.1002/ijc.2910450304. [DOI] [PubMed] [Google Scholar]
- Schor N. A., Cornelisse C. J. Biochemical and quantitative histochemical study of reduced pyridine nucleotide dehydrogenation by human colonic carcinomas. Cancer Res. 1983 Oct;43(10):4850–4855. [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Smitskamp-Wilms E., Giaccone G., Pinedo H. M., van der Laan B. F., Peters G. J. DT-diaphorase activity in normal and neoplastic human tissues; an indicator for sensitivity to bioreductive agents? Br J Cancer. 1995 Oct;72(4):917–921. doi: 10.1038/bjc.1995.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttie J. W. Vitamin K-dependent carboxylase. Annu Rev Biochem. 1985;54:459–477. doi: 10.1146/annurev.bi.54.070185.002331. [DOI] [PubMed] [Google Scholar]
- Tomasz M., Lipman R., Chowdary D., Pawlak J., Verdine G. L., Nakanishi K. Isolation and structure of a covalent cross-link adduct between mitomycin C and DNA. Science. 1987 Mar 6;235(4793):1204–1208. doi: 10.1126/science.3103215. [DOI] [PubMed] [Google Scholar]
- Wermuth B. Purification and properties of an NADPH-dependent carbonyl reductase from human brain. Relationship to prostaglandin 9-ketoreductase and xenobiotic ketone reductase. J Biol Chem. 1981 Feb 10;256(3):1206–1213. [PubMed] [Google Scholar]
- Workman P. Bioreductive mechanisms. Int J Radiat Oncol Biol Phys. 1992;22(4):631–637. doi: 10.1016/0360-3016(92)90493-2. [DOI] [PubMed] [Google Scholar]
- Workman P. Enzyme-directed bioreductive drug development revisited: a commentary on recent progress and future prospects with emphasis on quinone anticancer agents and quinone metabolizing enzymes, particularly DT-diaphorase. Oncol Res. 1994;6(10-11):461–475. [PubMed] [Google Scholar]
- Workman P., Stratford I. J. The experimental development of bioreductive drugs and their role in cancer therapy. Cancer Metastasis Rev. 1993 Jun;12(2):73–82. doi: 10.1007/BF00689802. [DOI] [PubMed] [Google Scholar]




