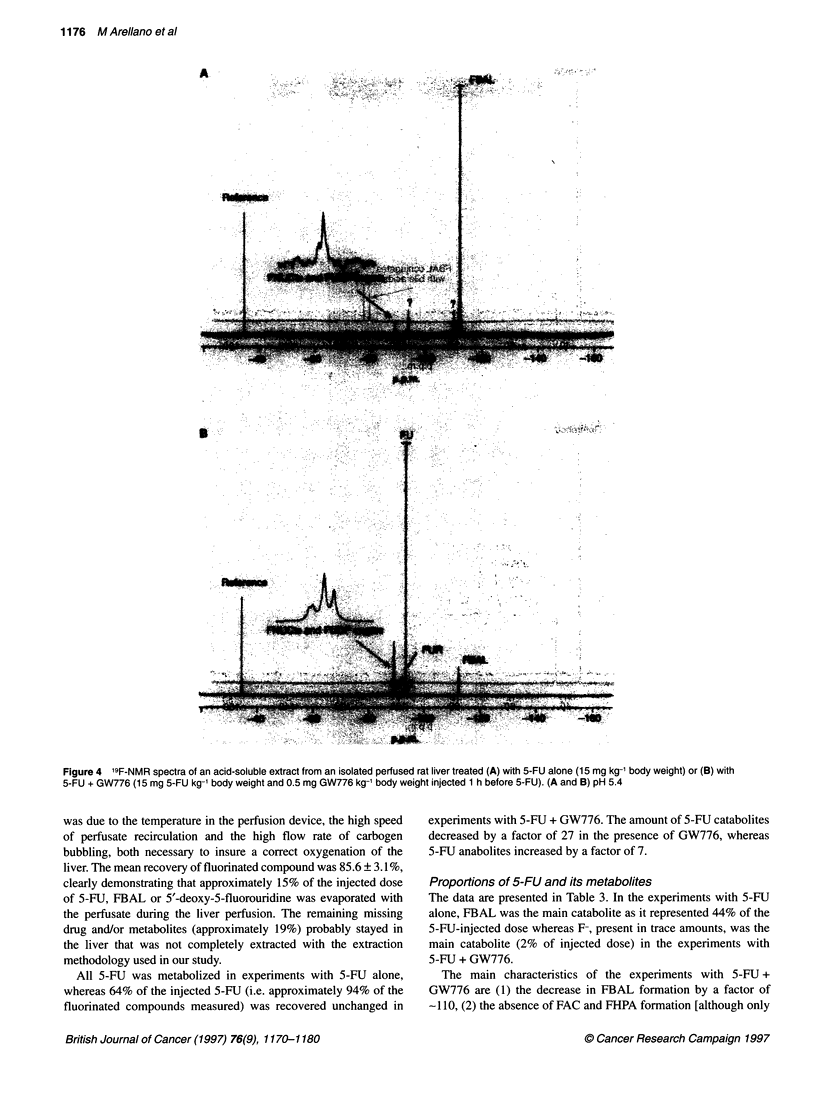
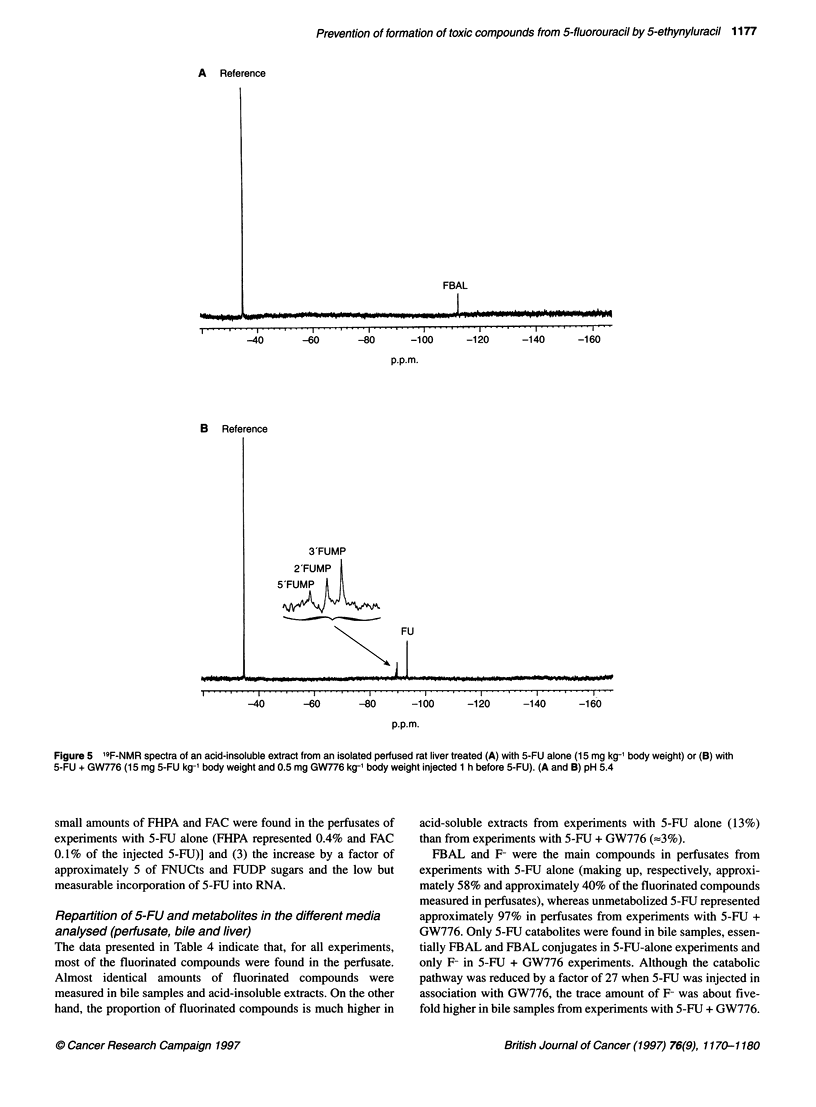
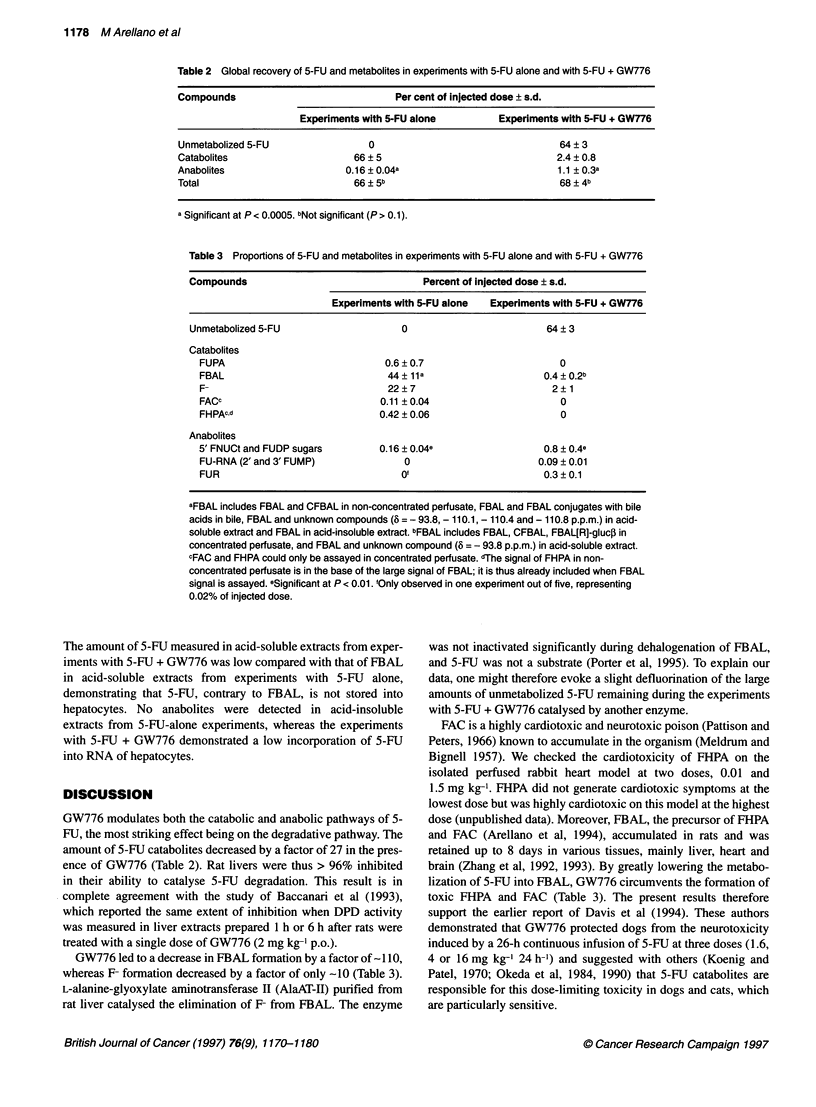
Abstract
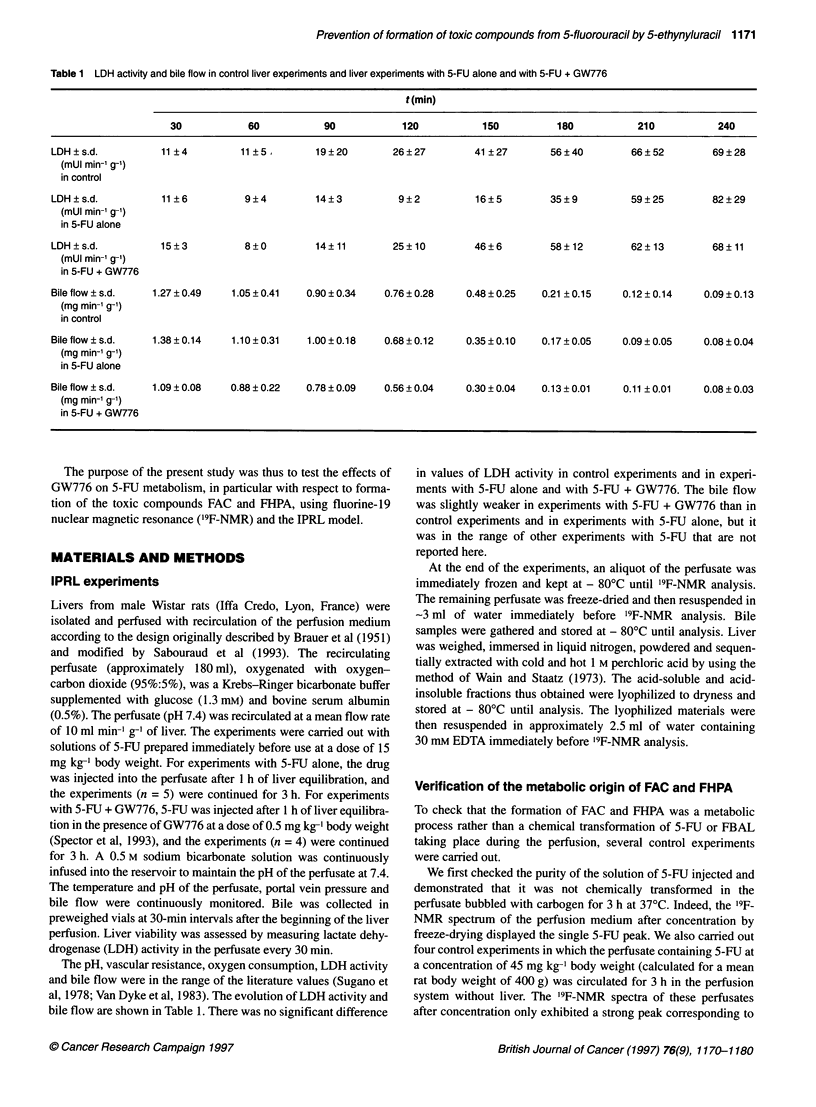
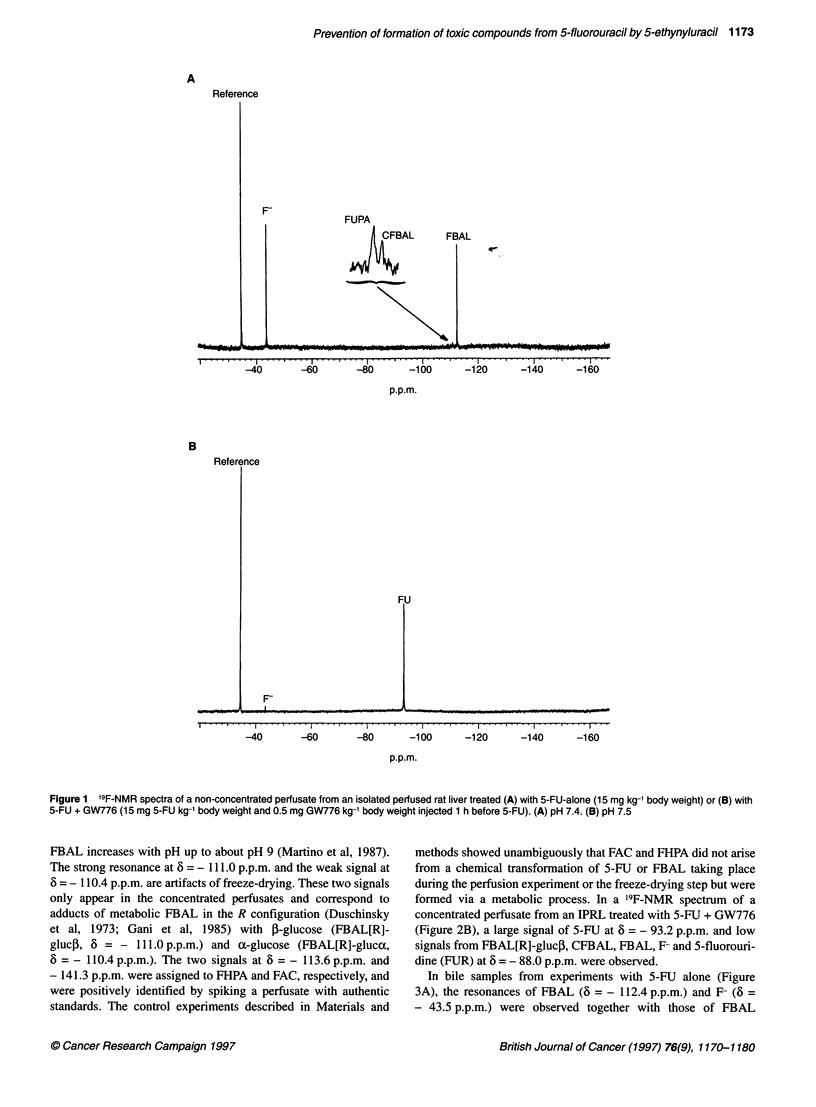
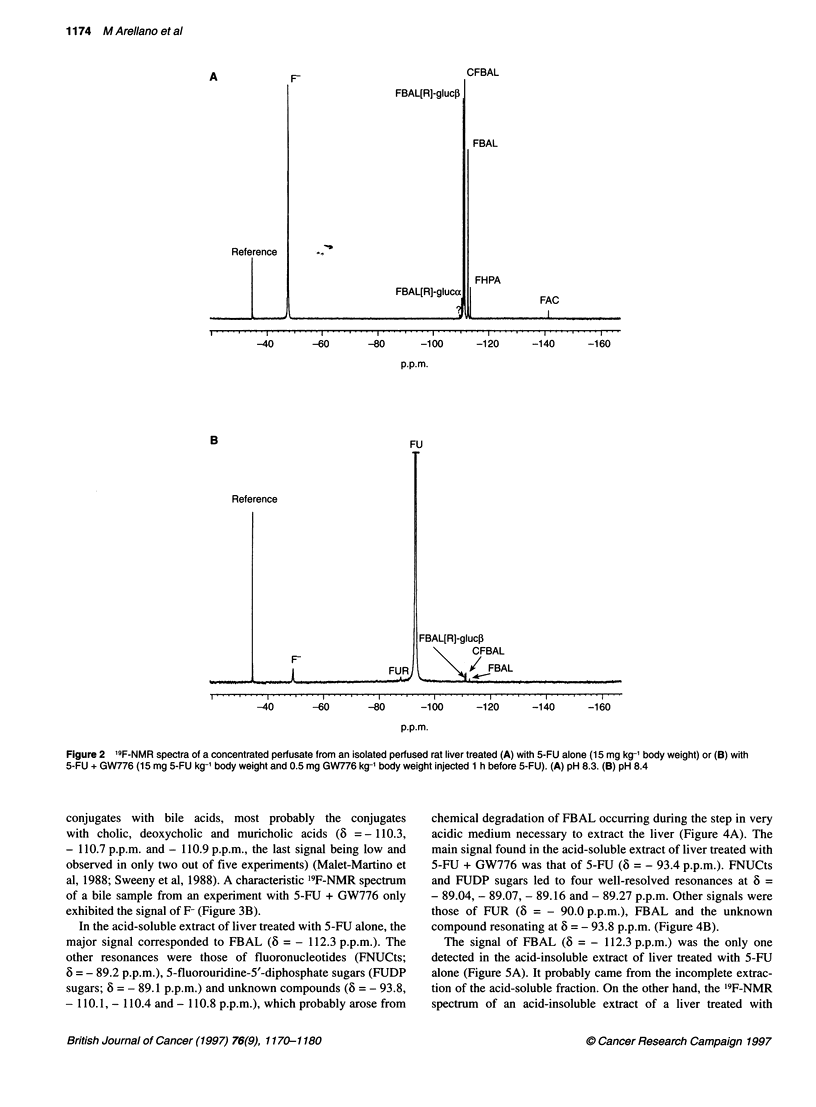
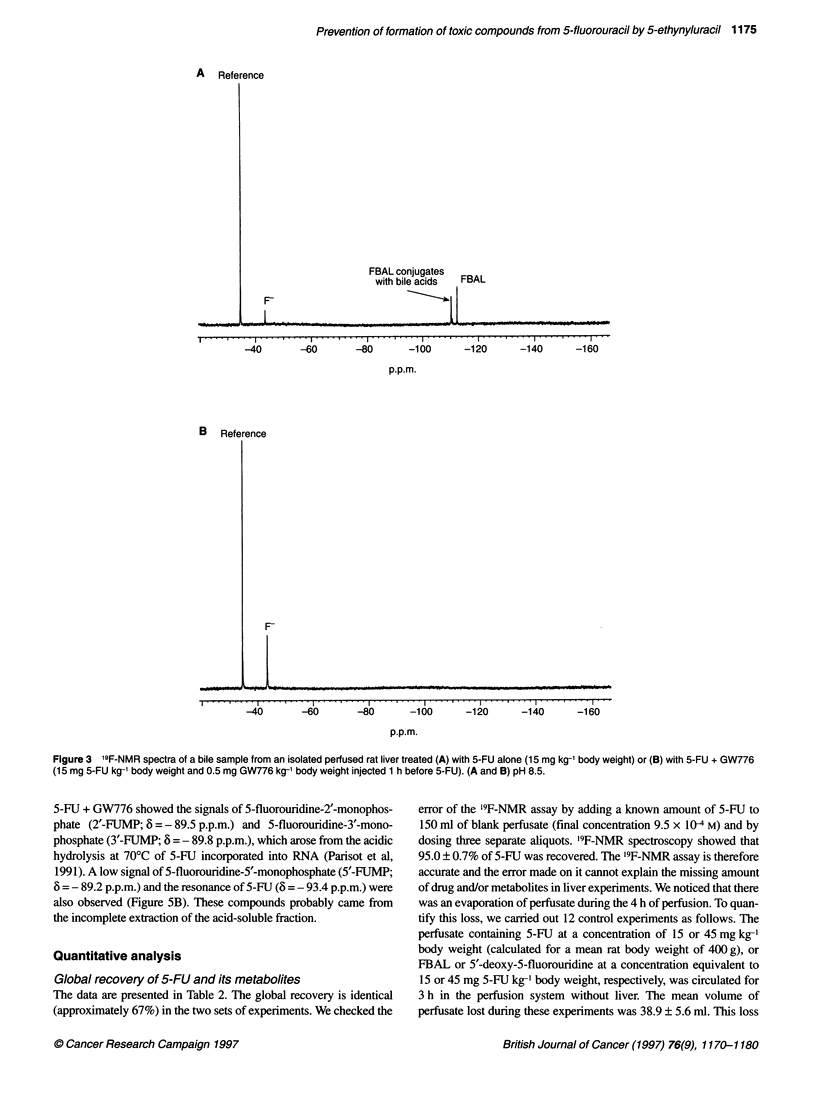
We studied the effects of 5-ethynyluracil (GW776), a potent inactivator of dihydropyrimidine dehydrogenase, on the metabolism of 5-fluorouracil (5-FU), in particular with respect to formation of the toxic compounds fluoroacetate (FAC) and 2-fluoro-3-hydroxypropionic acid (FHPA), using fluorine-19 nuclear magnetic resonance and the isolated perfused rat liver model. Livers were perfused with 5-FU alone at a dose of 15 mg kg(-1) body weight or with 5-FU + GW776 at doses of 15 mg 5-FU kg(-1) body weight and 0.5 mg GW776 kg(-1) body weight injected 1 h before 5-FU. All 5-FU was metabolized in experiments with 5-FU alone whereas unmetabolized 5-FU represented 94% of the fluorinated compounds measured in experiments with 5-FU + GW776. GW776 modulated both the catabolic and the anabolic pathways of 5-FU, the most striking effect being on the degradative pathway. The amount of 5-FU catabolites decreased by a factor of 27 in the presence of GW776. The modulator led to a decrease in alpha-fluoro-beta-alanine (FBAL) formation by a factor of approximately 110, while fluoride ion formation decreased by a factor of approximately 10. By strongly lowering the metabolism of 5-FU into FBAL, GW776 circumvented the transformation of FBAL into toxic FAC and FHPA. 5-FU anabolites increased by a factor of approximately 7 in the presence of GW776. The level of free fluoronucleotides and 5-fluorouridine-5'-diphosphate sugars was increased up to fivefold. No incorporation of 5-FU into RNA could be measured in experiments with 5-FU alone whereas, although low (0.1% of 5-FU injected dose), it was detectable in experiments with 5-FU + GW776. These results suggest that GW776 may be useful for attenuating the not very common but serious cardiotoxic and/or neurotoxic side-effects of 5-FU that are probably due to FBAL metabolites.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anand A. J. Fluorouracil cardiotoxicity. Ann Pharmacother. 1994 Mar;28(3):374–378. doi: 10.1177/106002809402800314. [DOI] [PubMed] [Google Scholar]
- BRAUER R. W., PESSOTTI R. L., PIZZOLATO P. Isolated rat liver preparation; bile production and other basic properties. Proc Soc Exp Biol Med. 1951 Oct;78(1):174–181. doi: 10.3181/00379727-78-19012. [DOI] [PubMed] [Google Scholar]
- Baccanari D. P., Davis S. T., Knick V. C., Spector T. 5-Ethynyluracil (776C85): a potent modulator of the pharmacokinetics and antitumor efficacy of 5-fluorouracil. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11064–11068. doi: 10.1073/pnas.90.23.11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernadou J., Armand J. P., Lopez A., Malet-Martino M. C., Martino R. Complete urinary excretion profile of 5-fluorouracil during a six-day chemotherapeutic schedule, as resolved by 19F nuclear magnetic resonance. Clin Chem. 1985 Jun;31(6):846–848. [PubMed] [Google Scholar]
- Cao S., Rustum Y. M., Spector T. 5-Ethynyluracil (776C85): modulation of 5-fluorouracil efficacy and therapeutic index in rats bearing advanced colorectal carcinoma. Cancer Res. 1994 Mar 15;54(6):1507–1510. [PubMed] [Google Scholar]
- Koenig H., Patel A. Biochemical basis for fluorouracil neurotoxicity. The role of Krebs cycle inhibition by fluoroacetate. Arch Neurol. 1970 Aug;23(2):155–160. doi: 10.1001/archneur.1970.00480260061008. [DOI] [PubMed] [Google Scholar]
- Lemaire L., Malet-Martino M. C., de Forni M., Martino R., Lasserre B. Cardiotoxicity of commercial 5-fluorouracil vials stems from the alkaline hydrolysis of this drug. Br J Cancer. 1992 Jul;66(1):119–127. doi: 10.1038/bjc.1992.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUKHERJEE K. L., HEIDELBERGER C. Studies on fluorinated pyrimidines. IX. The degradation of 5-fluorouracil-6-C14. J Biol Chem. 1960 Feb;235:433–437. [PubMed] [Google Scholar]
- Malet-Martino M. C., Bernadou J., Martino R., Armand J. P. 19F NMR spectrometry evidence for bile acid conjugates of alpha-fluoro-beta-alanine as the main biliary metabolites of antineoplastic fluoropyrimidines in humans. Drug Metab Dispos. 1988 Jan-Feb;16(1):78–84. [PubMed] [Google Scholar]
- Malet-Martino M. C., Martino R. Magnetic resonance spectroscopy: a powerful tool for drug metabolism studies. Biochimie. 1992 Sep-Oct;74(9-10):785–800. doi: 10.1016/0300-9084(92)90061-i. [DOI] [PubMed] [Google Scholar]
- Martino R., Lopez A., Malet-Martino M. C., Bernadou J., Armand J. P. Release of fluoride ion from 5'-deoxy-5-fluorouridine, an antineoplastic fluoropyrimidine, in humans. Drug Metab Dispos. 1985 Jan-Feb;13(1):116–118. [PubMed] [Google Scholar]
- Martino R., Malet-Martino M. C., Vialaneix C., Lopez A., Bon M. 19F nuclear magnetic resonance analysis of the carbamate reaction of alpha-fluoro-beta-alanine (FBAL), the major catabolite of fluoropyrimidines. Application to FBAL carbamate determination in body fluids of patients treated with 5'-deoxy-5-fluorouridine. Drug Metab Dispos. 1987 Nov-Dec;15(6):897–904. [PubMed] [Google Scholar]
- Okeda R., Karakama T., Kimura S., Toizumi S., Mitsushima T., Yokoyama Y. Neuropathologic study on chronic neurotoxicity of 5-fluorouracil and its masked compounds in dogs. Acta Neuropathol. 1984;63(4):334–343. doi: 10.1007/BF00687342. [DOI] [PubMed] [Google Scholar]
- Okeda R., Shibutani M., Matsuo T., Kuroiwa T., Shimokawa R., Tajima T. Experimental neurotoxicity of 5-fluorouracil and its derivatives is due to poisoning by the monofluorinated organic metabolites, monofluoroacetic acid and alpha-fluoro-beta-alanine. Acta Neuropathol. 1990;81(1):66–73. doi: 10.1007/BF00662639. [DOI] [PubMed] [Google Scholar]
- Parisot D., Malet-Martino M. C., Martino R., Crasnier P. F Nuclear Magnetic Resonance Analysis of 5-Fluorouracil Metabolism in Four Differently Pigmented Strains of Nectria haematococca. Appl Environ Microbiol. 1991 Dec;57(12):3605–3612. doi: 10.1128/aem.57.12.3605-3612.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter D. J., Chestnut W. G., Merrill B. M., Spector T. Mechanism-based inactivation of dihydropyrimidine dehydrogenase by 5-ethynyluracil. J Biol Chem. 1992 Mar 15;267(8):5236–5242. [PubMed] [Google Scholar]
- Porter D. J., Harrington J. A., Almond M. R., Chestnut W. G., Tanoury G., Spector T. Enzymatic elimination of fluoride from alpha-fluoro-beta-alanine. Biochem Pharmacol. 1995 Oct 26;50(9):1475–1484. doi: 10.1016/0006-2952(95)02053-5. [DOI] [PubMed] [Google Scholar]
- Sabouraud A., Redureau M., Gires P., Martinet M., Scherrmann J. M. Effect of colchicine-specific Fab fragments on the hepatic clearance of colchicine. Drug Metab Dispos. 1993 Nov-Dec;21(6):997–1002. [PubMed] [Google Scholar]
- Spector T., Harrington J. A., Porter D. J. 5-Ethynyluracil (776C85): inactivation of dihydropyrimidine dehydrogenase in vivo. Biochem Pharmacol. 1993 Dec 14;46(12):2243–2248. doi: 10.1016/0006-2952(93)90615-4. [DOI] [PubMed] [Google Scholar]
- Sugano T., Suda K., Shimada M., Oshino N. Biochemical and ultrastructural evaluation of isolated rat liver systems perfused with a hemoglobin-free medium. J Biochem. 1978 Apr;83(4):995–1007. doi: 10.1093/oxfordjournals.jbchem.a132028. [DOI] [PubMed] [Google Scholar]
- Sweeny D. J., Barnes S., Diasio R. B. Formation of conjugates of 2-fluoro-beta-alanine and bile acids during the metabolism of 5-fluorouracil and 5-fluoro-2-deoxyuridine in the isolated perfused rat liver. Cancer Res. 1988 Apr 15;48(8):2010–2014. [PubMed] [Google Scholar]
- Wain W. H., Staatz W. D. Rates of synthesis of ribosomal protein and total ribonucleic acid through the cell cycle of the fission yeast Schizosaccharomyces pombe. Exp Cell Res. 1973 Oct;81(2):269–278. doi: 10.1016/0014-4827(73)90515-6. [DOI] [PubMed] [Google Scholar]
- Yeh K. H., Cheng A. L. Acute confusion induced by a high-dose infusion of 5-fluorouracil and folinic acid. J Formos Med Assoc. 1994 Aug;93(8):721–723. [PubMed] [Google Scholar]
- Zhang R. W., Soong S. J., Liu T. P., Barnes S., Diasio S. B. Pharmacokinetics and tissue distribution of 2-fluoro-beta-alanine in rats. Potential relevance to toxicity pattern of 5-fluorouracil. Drug Metab Dispos. 1992 Jan-Feb;20(1):113–119. [PubMed] [Google Scholar]
- Zhang R., Liu T., Soong S. J., Diasio R. B. A mathematical model of the kinetics and tissue distribution of 2-fluoro-beta-alanine, the major catabolite of 5-fluorouracil. Biochem Pharmacol. 1993 May 25;45(10):2063–2069. doi: 10.1016/0006-2952(93)90017-q. [DOI] [PubMed] [Google Scholar]
- van Dyke R. W., Gollan J. L., Scharschmidt B. F. Oxygen consumption by rat liver: effects of taurocholate and sulfobromophthalein transport, glucagon, and cation substitution. Am J Physiol. 1983 May;244(5):G523–G531. doi: 10.1152/ajpgi.1983.244.5.G523. [DOI] [PubMed] [Google Scholar]


