Abstract
Ample evidence confirms that certain cancer cells have the capacity to produce multiple peptides as growth factors and that expression of their receptor may act in tumour cell paracrine and/or autocrine loop mechanisms, either by extracellular release of the growth factor or by the tumour itself. To study the possibility of an autocrine growth mechanism in bladder carcinoma, we investigated the ability of various bladder carcinoma cell lines to proliferate in serum-free medium. A rat bladder carcinoma cell line, BC47, demonstrated exponential and density-dependent growth in serum-free medium. Furthermore, conditioned medium from BC47 cells induced growth-stimulating activity for BC47 cells themselves. Purification and further characterization of this activity was performed by chromatographic methods, SDS-PAGE and N-terminal amino acid analysis. Finally, we have identified that a transferrin-like 70-kDa protein is found to be the main growth-promoting factor in this conditioned medium. In addition, specific antibodies against transferrin and the transferrin-receptor inhibit the in vitro growth of this cell line. Our data suggest that this transferrin-like factor possibly acts as an autocrine growth factor for cancer cells.
Full text
PDF

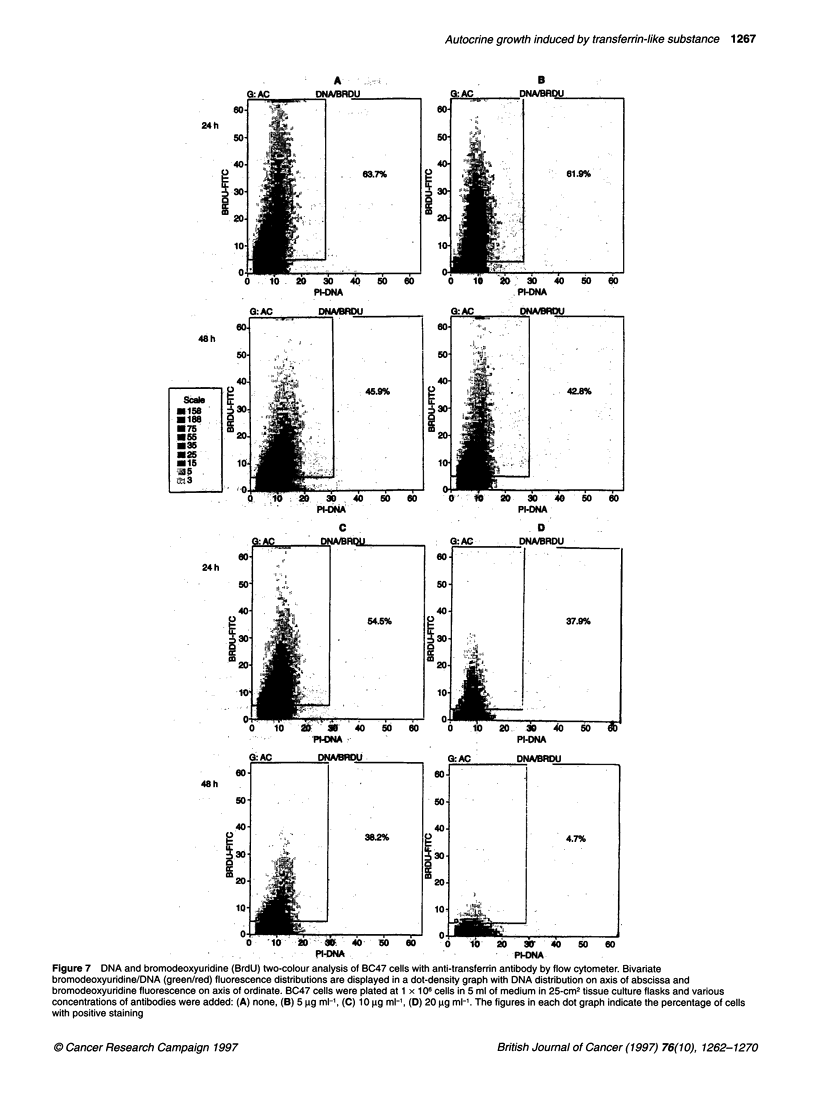
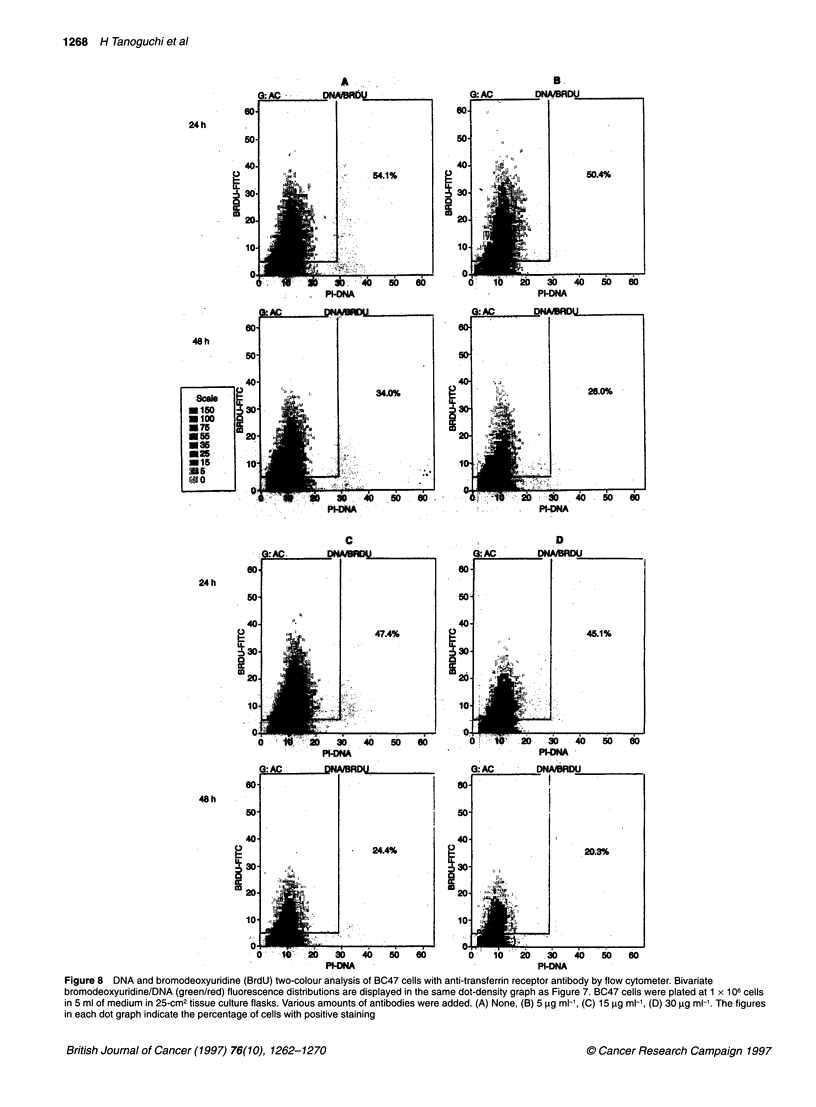
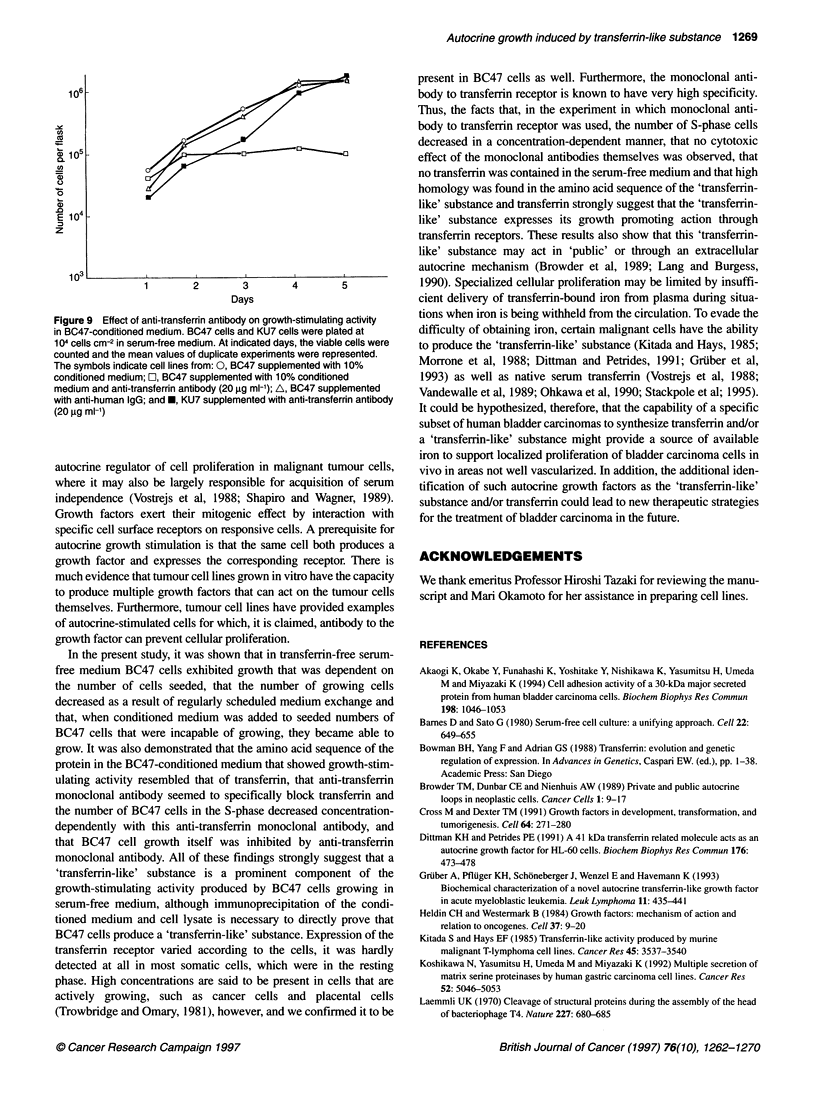

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaogi K., Okabe Y., Funahashi K., Yoshitake Y., Nishikawa K., Yasumitsu H., Umeda M., Miyazaki K. Cell adhesion activity of a 30-kDa major secreted protein from human bladder carcinoma cells. Biochem Biophys Res Commun. 1994 Feb 15;198(3):1046–1053. doi: 10.1006/bbrc.1994.1149. [DOI] [PubMed] [Google Scholar]
- Barnes D., Sato G. Serum-free cell culture: a unifying approach. Cell. 1980 Dec;22(3):649–655. doi: 10.1016/0092-8674(80)90540-1. [DOI] [PubMed] [Google Scholar]
- Bowman B. H., Yang F. M., Adrian G. S. Transferrin: evolution and genetic regulation of expression. Adv Genet. 1988;25:1–38. doi: 10.1016/s0065-2660(08)60457-5. [DOI] [PubMed] [Google Scholar]
- Browder T. M., Dunbar C. E., Nienhuis A. W. Private and public autocrine loops in neoplastic cells. Cancer Cells. 1989 Sep;1(1):9–17. [PubMed] [Google Scholar]
- Cross M., Dexter T. M. Growth factors in development, transformation, and tumorigenesis. Cell. 1991 Jan 25;64(2):271–280. doi: 10.1016/0092-8674(91)90638-f. [DOI] [PubMed] [Google Scholar]
- Dittmann K. H., Petrides P. E. A 41 kDa transferrin related molecule acts as an autocrine growth factor for HL-60 cells. Biochem Biophys Res Commun. 1991 Apr 15;176(1):473–478. doi: 10.1016/0006-291x(91)90948-7. [DOI] [PubMed] [Google Scholar]
- Grüber A., Pflüger K. H., Schöneberger J., Wenzel E., Havemann K. Biochemical characterization of a novel autocrine transferrin-like growth factor in acute myeloblastic leukemia. Leuk Lymphoma. 1993 Nov;11(5-6):435–441. doi: 10.3109/10428199309067937. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B. Growth factors: mechanism of action and relation to oncogenes. Cell. 1984 May;37(1):9–20. doi: 10.1016/0092-8674(84)90296-4. [DOI] [PubMed] [Google Scholar]
- Kitada S., Hays E. F. Transferrin-like activity produced by murine malignant T-lymphoma cell lines. Cancer Res. 1985 Aug;45(8):3537–3540. [PubMed] [Google Scholar]
- Koshikawa N., Yasumitsu H., Umeda M., Miyazaki K. Multiple secretion of matrix serine proteinases by human gastric carcinoma cell lines. Cancer Res. 1992 Sep 15;52(18):5046–5053. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lang R. A., Burgess A. W. Autocrine growth factors and tumourigenic transformation. Immunol Today. 1990 Jul;11(7):244–249. doi: 10.1016/0167-5699(90)90098-t. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Mandler R., Murano G., Katz D. A., Gordon R. K., Chiang P. K., Schiffmann E. Tumor cell autocrine motility factor. Proc Natl Acad Sci U S A. 1986 May;83(10):3302–3306. doi: 10.1073/pnas.83.10.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGillivray R. T., Mendez E., Shewale J. G., Sinha S. K., Lineback-Zins J., Brew K. The primary structure of human serum transferrin. The structures of seven cyanogen bromide fragments and the assembly of the complete structure. J Biol Chem. 1983 Mar 25;258(6):3543–3553. [PubMed] [Google Scholar]
- MacGillivray R. T., Mendez E., Sinha S. K., Sutton M. R., Lineback-Zins J., Brew K. The complete amino acid sequence of human serum transferrin. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2504–2508. doi: 10.1073/pnas.79.8.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda Y., Yoshitake Y., Nishikawa K. Growth control of A431 cells in protein-free medium: secretory products do not affect cell growth. In Vitro Cell Dev Biol. 1988 Sep;24(9):893–899. doi: 10.1007/BF02623899. [DOI] [PubMed] [Google Scholar]
- Matsuda H., Matsumoto M., Haraguchi S., Kanai K. Partial purification of a growth factor synthesized by a rat hepatoma cell line established in serum-free medium. Cancer Res. 1989 Apr 15;49(8):2118–2122. [PubMed] [Google Scholar]
- May W. S., Jr, Cuatrecasas P. Transferrin receptor: its biological significance. J Membr Biol. 1985;88(3):205–215. doi: 10.1007/BF01871086. [DOI] [PubMed] [Google Scholar]
- Messing E. M., Bubbers J. E., Dekernion J. B., Fahey J. L. Growth stimulating activity produced by human bladder cancer cells. J Urol. 1984 Dec;132(6):1230–1234. doi: 10.1016/s0022-5347(17)50111-1. [DOI] [PubMed] [Google Scholar]
- Messing E. M., Fahey J. L., deKernion J. B., Bhuta S. M., Bubbers J. E. Serum-free medium for the in vitro growth of normal and malignant urinary bladder epithelial cells. Cancer Res. 1982 Jun;42(6):2392–2397. [PubMed] [Google Scholar]
- Morrone G., Corbo L., Turco M. C., Pizzano R., De Felice M., Bridges S., Venuta S. Transferrin-like autocrine growth factor, derived from T-lymphoma cells, that inhibits normal T-cell proliferation. Cancer Res. 1988 Jun 15;48(12):3425–3429. [PubMed] [Google Scholar]
- Rodeck U., Herlyn M. Growth factors in melanoma. Cancer Metastasis Rev. 1991 Jun;10(2):89–101. doi: 10.1007/BF00049407. [DOI] [PubMed] [Google Scholar]
- Shapiro L. E., Wagner N. Transferrin is an autocrine growth factor secreted by Reuber H-35 cells in serum-free culture. In Vitro Cell Dev Biol. 1989 Jul;25(7):650–654. doi: 10.1007/BF02623636. [DOI] [PubMed] [Google Scholar]
- Shewale J. G., Brew K. Effects of Fe3+ binding on the microenvironments of individual amino groups in human serum transferrin as determined by differential kinetic labeling. J Biol Chem. 1982 Aug 25;257(16):9406–9415. [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Autocrine growth factors and cancer. 1985 Feb 28-Mar 6Nature. 313(6005):745–747. doi: 10.1038/313745a0. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Todaro G. J. Autocrine secretion and malignant transformation of cells. N Engl J Med. 1980 Oct 9;303(15):878–880. doi: 10.1056/NEJM198010093031511. [DOI] [PubMed] [Google Scholar]
- Stackpole C. W., Kalbag S. S., Groszek L. Acquisition of in vitro growth autonomy during B16 melanoma malignant progression is associated with autocrine stimulation by transferrin and fibronectin. In Vitro Cell Dev Biol Anim. 1995 Mar;31(3):244–251. doi: 10.1007/BF02639440. [DOI] [PubMed] [Google Scholar]
- Tachibana M., Miyakawa A., Tazaki H., Nakamura K., Kubo A., Hata J., Nishi T., Amano Y. Autocrine growth of transitional cell carcinoma of the bladder induced by granulocyte-colony stimulating factor. Cancer Res. 1995 Aug 1;55(15):3438–3443. [PubMed] [Google Scholar]
- Tachibana M. Studies on cellular adhesiveness in five different culture cell lines derived from carcinoma of the urinary bladder. Keio J Med. 1982 Oct;31(3):127–148. doi: 10.2302/kjm.31.127. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Omary M. B. Human cell surface glycoprotein related to cell proliferation is the receptor for transferrin. Proc Natl Acad Sci U S A. 1981 May;78(5):3039–3043. doi: 10.1073/pnas.78.5.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandewalle B., Hornez L., Revillion F., Lefebvre J. Secretion of transferrin by human breast cancer cells. Biochem Biophys Res Commun. 1989 Aug 30;163(1):149–154. doi: 10.1016/0006-291x(89)92112-8. [DOI] [PubMed] [Google Scholar]
- Vostrejs M., Moran P. L., Seligman P. A. Transferrin synthesis by small cell lung cancer cells acts as an autocrine regulator of cellular proliferation. J Clin Invest. 1988 Jul;82(1):331–339. doi: 10.1172/JCI113591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R. S., Passaro E., Jr Growth factors, oncogenes and the autocrine hypothesis. Surg Gynecol Obstet. 1989 May;168(5):468–473. [PubMed] [Google Scholar]
- Yang F., Lum J. B., McGill J. R., Moore C. M., Naylor S. L., van Bragt P. H., Baldwin W. D., Bowman B. H. Human transferrin: cDNA characterization and chromosomal localization. Proc Natl Acad Sci U S A. 1984 May;81(9):2752–2756. doi: 10.1073/pnas.81.9.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]