Abstract
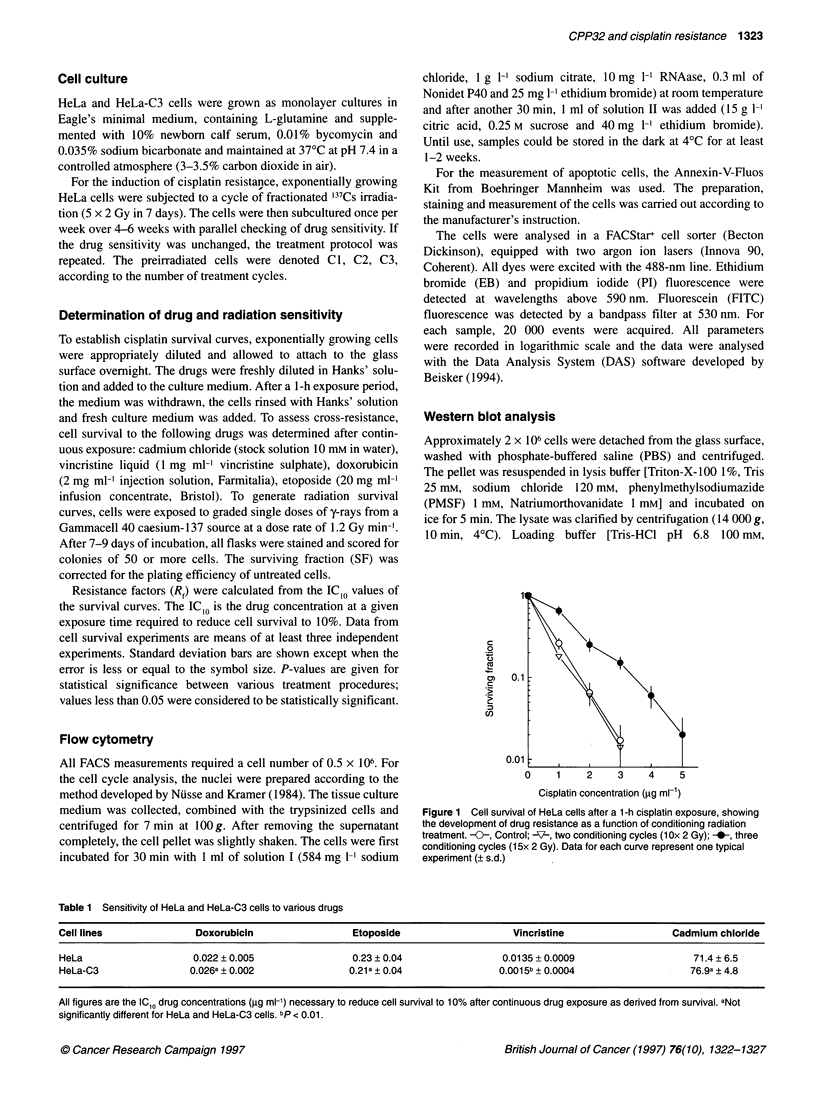
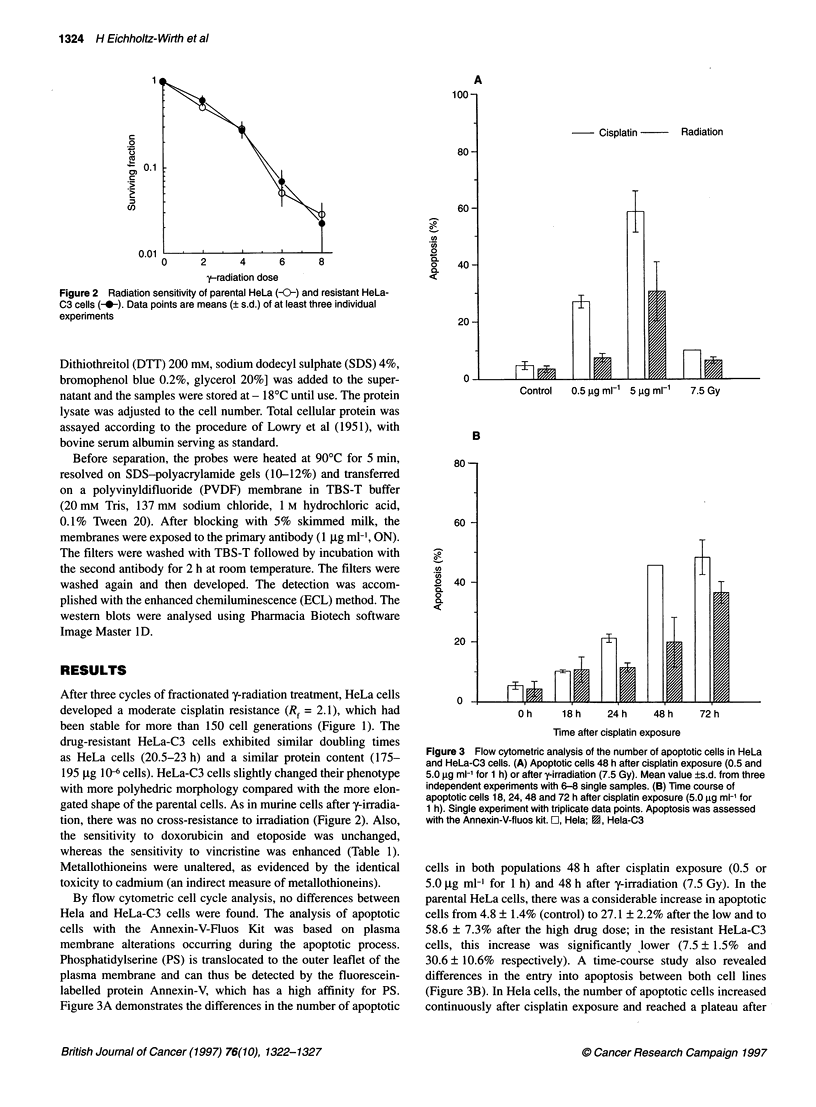
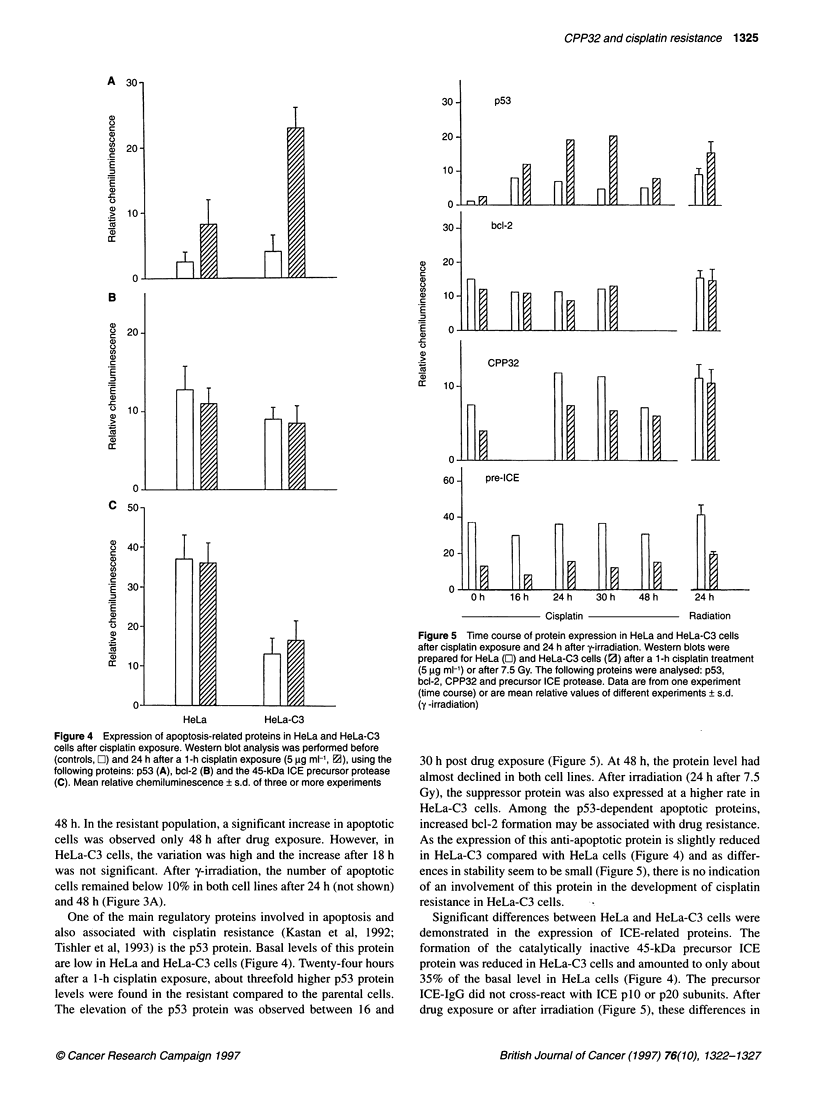
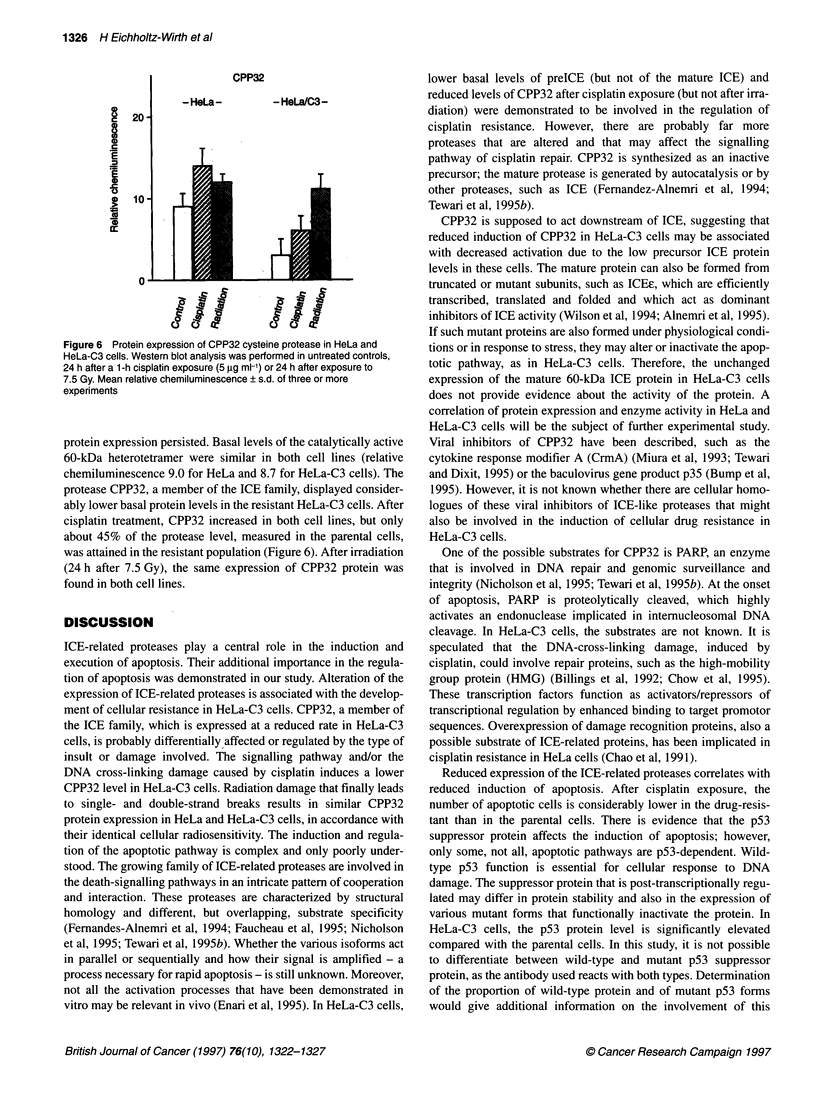
Low-dose fractionated gamma-irradiation (three cycles of 5 x 2 Gy) induced cisplatin resistance in HeLa cells. The drug resistance was modest (Rf of about 2) and stable, similar to that found previously in murine cells after irradiation. In the drug-resistant HeLa-C3 cells, flow cytometric analysis revealed a decreased number of apoptotic cells compared with the parental cells. Drug resistance was associated with considerably enhanced expression of the p53 suppressor protein in HeLa-C3 cells after cisplatin exposure but seemed not to be regulated by the bcl-2-dependent pathway. Cisplatin resistance correlated with reduced expression of ICE-related proteases (interleukin-1beta-converting enzyme). Basal levels of the 45-kDa precursor ICE protein were reduced in HeLa-C3 cells, while those of the mature 60-kDa heterotetramer were similar. The CPP32 protease, a member of the ICE family with structural homology but different substrate specificity, was expressed at a lowered level. After drug exposure, there was a slight increase of CPP32 in HeLa-C3 cells, equivalent to about 45% of the level attained in the parental cells. This is in contrast to the CPP32 levels measured after irradiation, which were similar in sensitive and in resistant cells. As the radiosensitivity is unchanged in both cell lines, these results suggest that cisplatin resistance in HeLa-C3 cells is associated with alterations of a CPP32-linked apoptotic pathway, which is affected by the damage caused by cisplatin but not by irradiation. Whether these changes are dependent on the observed p53 modifications is now being studied in resistant clones.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alnemri E. S., Fernandes-Alnemri T., Litwack G. Cloning and expression of four novel isoforms of human interleukin-1 beta converting enzyme with different apoptotic activities. J Biol Chem. 1995 Mar 3;270(9):4312–4317. doi: 10.1074/jbc.270.9.4312. [DOI] [PubMed] [Google Scholar]
- Beisker W. A new combined integral-light and slit-scan data analysis system (DAS) for flow cytometry. Comput Methods Programs Biomed. 1994 Feb 1;42(1):15–26. doi: 10.1016/0169-2607(94)90134-1. [DOI] [PubMed] [Google Scholar]
- Billings P. C., Davis R. J., Engelsberg B. N., Skov K. A., Hughes E. N. Characterization of high mobility group protein binding to cisplatin-damaged DNA. Biochem Biophys Res Commun. 1992 Nov 16;188(3):1286–1294. doi: 10.1016/0006-291x(92)91371-v. [DOI] [PubMed] [Google Scholar]
- Bump N. J., Hackett M., Hugunin M., Seshagiri S., Brady K., Chen P., Ferenz C., Franklin S., Ghayur T., Li P. Inhibition of ICE family proteases by baculovirus antiapoptotic protein p35. Science. 1995 Sep 29;269(5232):1885–1888. doi: 10.1126/science.7569933. [DOI] [PubMed] [Google Scholar]
- Chao C. C., Huang S. L., Lee L. Y., Lin-Chao S. Identification of inducible damage-recognition proteins that are overexpressed in HeLa cells resistant to cis-diamminedichloroplatinum (II). Biochem J. 1991 Aug 1;277(Pt 3):875–878. doi: 10.1042/bj2770875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Smith M. R., Thirumalai K., Zychlinsky A. A bacterial invasin induces macrophage apoptosis by binding directly to ICE. EMBO J. 1996 Aug 1;15(15):3853–3860. [PMC free article] [PubMed] [Google Scholar]
- Chow C. S., Barnes C. M., Lippard S. J. A single HMG domain in high-mobility group 1 protein binds to DNAs as small as 20 base pairs containing the major cisplatin adduct. Biochemistry. 1995 Mar 7;34(9):2956–2964. doi: 10.1021/bi00009a027. [DOI] [PubMed] [Google Scholar]
- Eichholtz-Wirth H., Hietel B. Cisplatin resistance in mouse fibrosarcoma cells after low-dose irradiation in vitro and in vivo. Br J Cancer. 1994 Oct;70(4):579–584. doi: 10.1038/bjc.1994.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichholtz-Wirth H., Reidel G., Hietel B. Radiation-induced transient cisplatin resistance in murine fibrosarcoma cells associated with elevated metallothionein content. Br J Cancer. 1993 May;67(5):1001–1006. doi: 10.1038/bjc.1993.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichholtz-Wirth H. Reversal of radiation-induced cisplatin resistance in murine fibrosarcoma cells by selective modulation of the cyclic GMP-dependent transduction pathway. Br J Cancer. 1995 Aug;72(2):287–292. doi: 10.1038/bjc.1995.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enari M., Hug H., Nagata S. Involvement of an ICE-like protease in Fas-mediated apoptosis. Nature. 1995 May 4;375(6526):78–81. doi: 10.1038/375078a0. [DOI] [PubMed] [Google Scholar]
- Faucheu C., Diu A., Chan A. W., Blanchet A. M., Miossec C., Hervé F., Collard-Dutilleul V., Gu Y., Aldape R. A., Lippke J. A. A novel human protease similar to the interleukin-1 beta converting enzyme induces apoptosis in transfected cells. EMBO J. 1995 May 1;14(9):1914–1922. doi: 10.1002/j.1460-2075.1995.tb07183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes-Alnemri T., Litwack G., Alnemri E. S. CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. J Biol Chem. 1994 Dec 9;269(49):30761–30764. [PubMed] [Google Scholar]
- Kastan M. B., Onyekwere O., Sidransky D., Vogelstein B., Craig R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991 Dec 1;51(23 Pt 1):6304–6311. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miura M., Zhu H., Rotello R., Hartwieg E. A., Yuan J. Induction of apoptosis in fibroblasts by IL-1 beta-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell. 1993 Nov 19;75(4):653–660. doi: 10.1016/0092-8674(93)90486-a. [DOI] [PubMed] [Google Scholar]
- Nicholson D. W., Ali A., Thornberry N. A., Vaillancourt J. P., Ding C. K., Gallant M., Gareau Y., Griffin P. R., Labelle M., Lazebnik Y. A. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995 Jul 6;376(6535):37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- Nüsse M., Kramer J. Flow cytometric analysis of micronuclei found in cells after irradiation. Cytometry. 1984 Jan;5(1):20–25. doi: 10.1002/cyto.990050105. [DOI] [PubMed] [Google Scholar]
- Tewari M., Beidler D. R., Dixit V. M. CrmA-inhibitable cleavage of the 70-kDa protein component of the U1 small nuclear ribonucleoprotein during Fas- and tumor necrosis factor-induced apoptosis. J Biol Chem. 1995 Aug 11;270(32):18738–18741. doi: 10.1074/jbc.270.32.18738. [DOI] [PubMed] [Google Scholar]
- Tewari M., Dixit V. M. Fas- and tumor necrosis factor-induced apoptosis is inhibited by the poxvirus crmA gene product. J Biol Chem. 1995 Feb 17;270(7):3255–3260. doi: 10.1074/jbc.270.7.3255. [DOI] [PubMed] [Google Scholar]
- Thornberry N. A., Bull H. G., Calaycay J. R., Chapman K. T., Howard A. D., Kostura M. J., Miller D. K., Molineaux S. M., Weidner J. R., Aunins J. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992 Apr 30;356(6372):768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- Tishler R. B., Calderwood S. K., Coleman C. N., Price B. D. Increases in sequence specific DNA binding by p53 following treatment with chemotherapeutic and DNA damaging agents. Cancer Res. 1993 May 15;53(10 Suppl):2212–2216. [PubMed] [Google Scholar]
- Wang L., Miura M., Bergeron L., Zhu H., Yuan J. Ich-1, an Ice/ced-3-related gene, encodes both positive and negative regulators of programmed cell death. Cell. 1994 Sep 9;78(5):739–750. doi: 10.1016/s0092-8674(94)90422-7. [DOI] [PubMed] [Google Scholar]
- Wilson K. P., Black J. A., Thomson J. A., Kim E. E., Griffith J. P., Navia M. A., Murcko M. A., Chambers S. P., Aldape R. A., Raybuck S. A. Structure and mechanism of interleukin-1 beta converting enzyme. Nature. 1994 Jul 28;370(6487):270–275. doi: 10.1038/370270a0. [DOI] [PubMed] [Google Scholar]


