Abstract
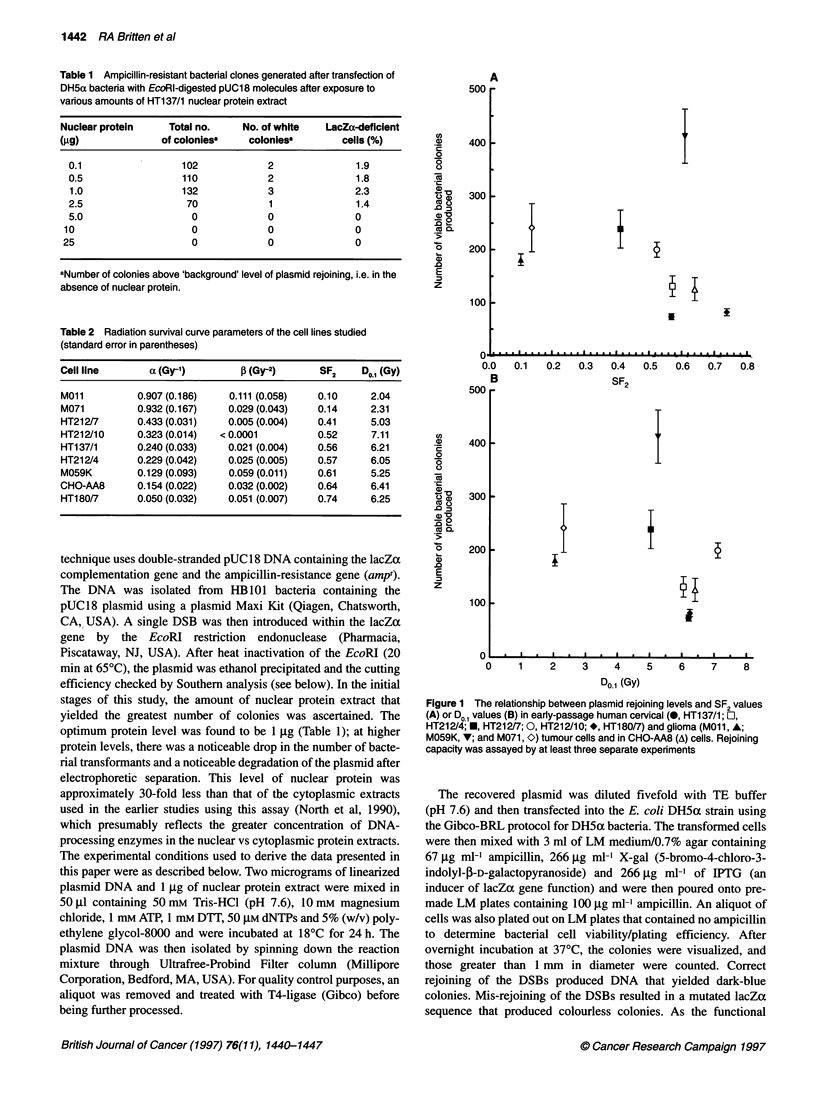
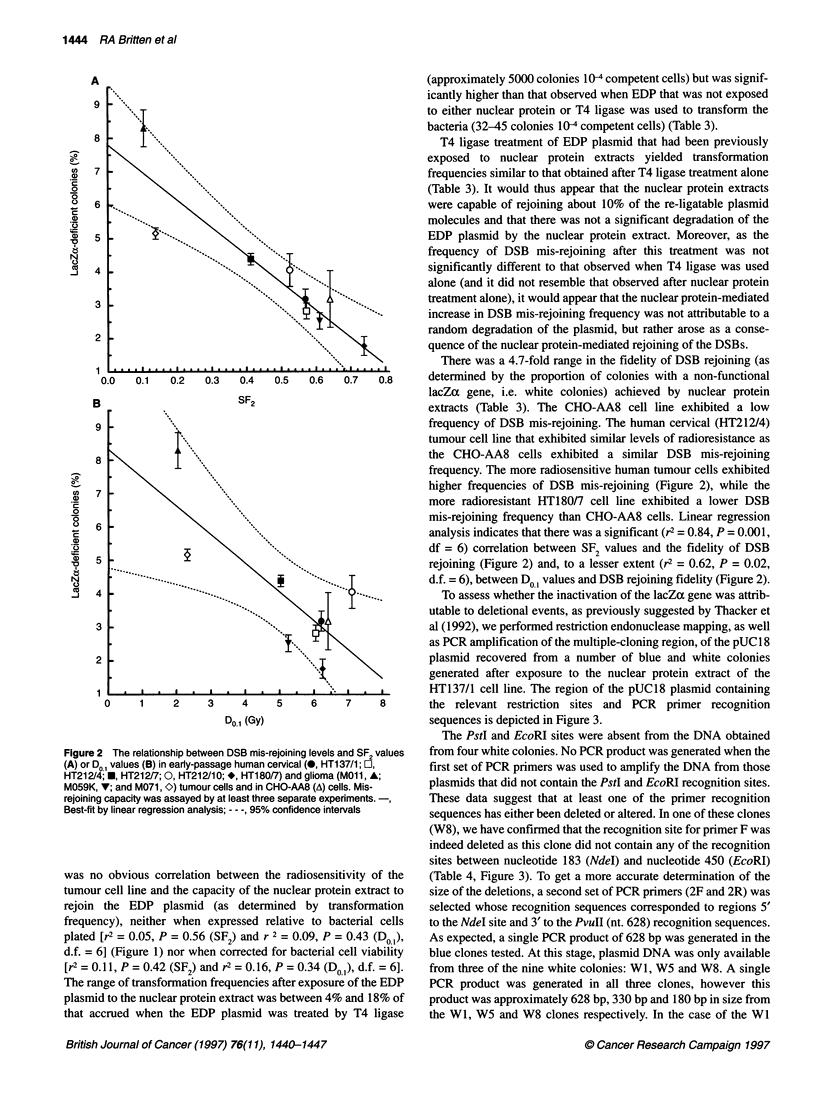
A cell-free plasmid reactivation assay was used to determine the fidelity of DNA double-strand break (DSB) repair in a panel of eight DSB repair-proficient human tumour cell lines. Nuclear protein extracts derived from radiosensitive tumour cells were less capable of correctly rejoining EcoRI-induced DSBs than were similar extracts from radioresistant tumour cells. Linear regression analysis suggests that there was a significant (r2 = 0.84, P = 0.001, d.f. = 6) correlation between the fidelity of DSB rejoining and the SF2 values of the cell lines studied. This cell-free assay is clearly sensitive to differences in the nuclear protein composition that reflect the clinically relevant radiosensitivity of these cell lines. The fact that our cell-free assay yielded similar results to previous studies that used intracellular plasmid reactivation assays suggests that those differences in DSB mis-rejoining frequencies in radiosensitive and radioresistant cell lines may be due to inherent differences in nuclear protein composition and are not directly attributable to differences in proliferation rates between cell lines. The underlying cause for this association between DSB mis-rejoining frequencies and radiosensitivity is presently unknown, however restriction endonuclease mapping and polymerase chain reaction (PCR) amplification analysis revealed that approximately 40% of the mis-rejoined DSBs arose as a result of the deletion of between 40 and 440 base pairs. These data raise the possibility that the radiosensitivity of DSB repair-proficient human tumour cell lines may be partly determined by the predisposition of these cell lines to activate non-conservative DSB rejoining pathways.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allalunis-Turner M. J., Barron G. M., Day R. S., 3rd, Fulton D. S., Urtasun R. C. Radiosensitivity testing of human primary brain tumor specimens. Int J Radiat Oncol Biol Phys. 1992;23(2):339–343. doi: 10.1016/0360-3016(92)90751-3. [DOI] [PubMed] [Google Scholar]
- Allalunis-Turner M. J., Lintott L. G., Barron G. M., Day R. S., 3rd, Lees-Miller S. P. Lack of correlation between DNA-dependent protein kinase activity and tumor cell radiosensitivity. Cancer Res. 1995 Nov 15;55(22):5200–5202. [PubMed] [Google Scholar]
- Allalunis-Turner M. J., Pearcey R. G., Barron G. M., Buryn D. A., Babiak J. C., Honoré L. H. Inherent radiosensitivity testing of tumor biopsies obtained from patients with carcinoma of the cervix or endometrium. Radiother Oncol. 1991 Nov;22(3):201–205. doi: 10.1016/0167-8140(91)90025-c. [DOI] [PubMed] [Google Scholar]
- Britten R. A., Evans A. J., Allalunis-Turner M. J., Franko A. J., Pearcey R. G. Intratumoral heterogeneity as a confounding factor in clonogenic assays for tumour radioresponsiveness. Radiother Oncol. 1996 May;39(2):145–153. doi: 10.1016/0167-8140(96)01719-7. [DOI] [PubMed] [Google Scholar]
- Britten R. A., Evans A. J., Allalunis-Turner M. J., Pearcey R. G. Effect of cisplatin on the clinically relevant radiosensitivity of human cervical carcinoma cell lines. Int J Radiat Oncol Biol Phys. 1996 Jan 15;34(2):367–374. doi: 10.1016/0360-3016(95)02088-8. [DOI] [PubMed] [Google Scholar]
- Bryant P. E., Liu N. Responses of radiosensitive repair-proficient cell lines to restriction endonucleases. Int J Radiat Biol. 1994 Nov;66(5):597–601. doi: 10.1080/09553009414551681. [DOI] [PubMed] [Google Scholar]
- Cox R., Masson W. K., Debenham P. G., Webb M. B. The use of recombinant DNA plasmids for the determination of DNA-repair and recombination in cultured mammalian cells. Br J Cancer Suppl. 1984;6:67–72. [PMC free article] [PubMed] [Google Scholar]
- Deacon J., Peckham M. J., Steel G. G. The radioresponsiveness of human tumours and the initial slope of the cell survival curve. Radiother Oncol. 1984 Dec;2(4):317–323. doi: 10.1016/s0167-8140(84)80074-2. [DOI] [PubMed] [Google Scholar]
- Fairman M. P., Johnson A. P., Thacker J. Multiple components are involved in the efficient joining of double stranded DNA breaks in human cell extracts. Nucleic Acids Res. 1992 Aug 25;20(16):4145–4152. doi: 10.1093/nar/20.16.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertil B., Malaise E. P. Inherent cellular radiosensitivity as a basic concept for human tumor radiotherapy. Int J Radiat Oncol Biol Phys. 1981 May;7(5):621–629. doi: 10.1016/0360-3016(81)90377-1. [DOI] [PubMed] [Google Scholar]
- Ganesh A., North P., Thacker J. Repair and misrepair of site-specific DNA double-strand breaks by human cell extracts. Mutat Res. 1993 May;299(3-4):251–259. doi: 10.1016/0165-1218(93)90101-i. [DOI] [PubMed] [Google Scholar]
- Giaccia A. J., Schwartz J., Shieh J., Brown J. M. The use of asymmetric-field inversion gel electrophoresis to predict tumor cell radiosensitivity. Radiother Oncol. 1992 Aug;24(4):231–238. doi: 10.1016/0167-8140(92)90229-n. [DOI] [PubMed] [Google Scholar]
- Giaccia A., Weinstein R., Hu J., Stamato T. D. Cell cycle-dependent repair of double-strand DNA breaks in a gamma-ray-sensitive Chinese hamster cell. Somat Cell Mol Genet. 1985 Sep;11(5):485–491. doi: 10.1007/BF01534842. [DOI] [PubMed] [Google Scholar]
- Johnston P. J., Bryant P. E. A component of DNA double-strand break repair is dependent on the spatial orientation of the lesions within the higher-order structures of chromatin. Int J Radiat Biol. 1994 Nov;66(5):531–536. doi: 10.1080/09553009414551571. [DOI] [PubMed] [Google Scholar]
- Kirchgessner C. U., Patil C. K., Evans J. W., Cuomo C. A., Fried L. M., Carter T., Oettinger M. A., Brown J. M. DNA-dependent kinase (p350) as a candidate gene for the murine SCID defect. Science. 1995 Feb 24;267(5201):1178–1183. doi: 10.1126/science.7855601. [DOI] [PubMed] [Google Scholar]
- Lambin P., Coco-Martin J., Legal J. D., Begg A. C., Parmentier C., Joiner M. C., Malaise E. P. Intrinsic radiosensitivity and chromosome aberration analysis using fluorescence in situ hybridization in cells of two human tumor cell lines. Radiat Res. 1994 Apr;138(1 Suppl):S40–S43. [PubMed] [Google Scholar]
- Lees-Miller S. P., Godbout R., Chan D. W., Weinfeld M., Day R. S., 3rd, Barron G. M., Allalunis-Turner J. Absence of p350 subunit of DNA-activated protein kinase from a radiosensitive human cell line. Science. 1995 Feb 24;267(5201):1183–1185. doi: 10.1126/science.7855602. [DOI] [PubMed] [Google Scholar]
- Liu N., Bryant P. E. Response of ataxia telangiectasia cells to restriction endonuclease induced DNA double-strand breaks: I. Cytogenetic characterization. Mutagenesis. 1993 Nov;8(6):503–510. doi: 10.1093/mutage/8.6.503. [DOI] [PubMed] [Google Scholar]
- Luo C. M., Tang W., Mekeel K. L., DeFrank J. S., Anné P. R., Powell S. N. High frequency and error-prone DNA recombination in ataxia telangiectasia cell lines. J Biol Chem. 1996 Feb 23;271(8):4497–4503. doi: 10.1074/jbc.271.8.4497. [DOI] [PubMed] [Google Scholar]
- North P., Ganesh A., Thacker J. The rejoining of double-strand breaks in DNA by human cell extracts. Nucleic Acids Res. 1990 Nov 11;18(21):6205–6210. doi: 10.1093/nar/18.21.6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive P. L., Banáth J. P., MacPhail H. S. Lack of a correlation between radiosensitivity and DNA double-strand break induction or rejoining in six human tumor cell lines. Cancer Res. 1994 Jul 15;54(14):3939–3946. [PubMed] [Google Scholar]
- Olnes M. I., Kurl R. N. Isolation of nuclear extracts from fragile cells: a simplified procedure applied to thymocytes. Biotechniques. 1994 Nov;17(5):828–829. [PubMed] [Google Scholar]
- Powell S. N., McMillan T. J. The repair fidelity of restriction enzyme-induced double strand breaks in plasmid DNA correlates with radioresistance in human tumor cell lines. Int J Radiat Oncol Biol Phys. 1994 Jul 30;29(5):1035–1040. doi: 10.1016/0360-3016(94)90399-9. [DOI] [PubMed] [Google Scholar]
- Powell S. N., Whitaker S. J., Edwards S. M., McMillan T. J. A DNA repair defect in a radiation-sensitive clone of a human bladder carcinoma cell line. Br J Cancer. 1992 Jun;65(6):798–802. doi: 10.1038/bjc.1992.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell S., McMillan T. J. Clonal variation of DNA repair in a human glioma cell line. Radiother Oncol. 1991 Aug;21(4):225–232. doi: 10.1016/0167-8140(91)90046-j. [DOI] [PubMed] [Google Scholar]
- Powell S., Whitaker S., Peacock J., McMillan T. Ataxia telangiectasia: an investigation of the repair defect in the cell line AT5BIVA by plasmid reconstitution. Mutat Res. 1993 Jun;294(1):9–20. doi: 10.1016/0921-8777(93)90053-j. [DOI] [PubMed] [Google Scholar]
- Russell N. S., Arlett C. F., Bartelink H., Begg A. C. Use of fluorescence in situ hybridization to determine the relationship between chromosome aberrations and cell survival in eight human fibroblast strains. Int J Radiat Biol. 1995 Aug;68(2):185–196. doi: 10.1080/09553009514551091. [DOI] [PubMed] [Google Scholar]
- Sasai K., Evans J. W., Kovacs M. S., Brown J. M. Prediction of human cell radiosensitivity: comparison of clonogenic assay with chromosome aberrations scored using premature chromosome condensation with fluorescence in situ hybridization. Int J Radiat Oncol Biol Phys. 1994 Dec 1;30(5):1127–1132. doi: 10.1016/0360-3016(94)90319-0. [DOI] [PubMed] [Google Scholar]
- Savitsky K., Bar-Shira A., Gilad S., Rotman G., Ziv Y., Vanagaite L., Tagle D. A., Smith S., Uziel T., Sfez S. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science. 1995 Jun 23;268(5218):1749–1753. doi: 10.1126/science.7792600. [DOI] [PubMed] [Google Scholar]
- Taccioli G. E., Gottlieb T. M., Blunt T., Priestley A., Demengeot J., Mizuta R., Lehmann A. R., Alt F. W., Jackson S. P., Jeggo P. A. Ku80: product of the XRCC5 gene and its role in DNA repair and V(D)J recombination. Science. 1994 Sep 2;265(5177):1442–1445. doi: 10.1126/science.8073286. [DOI] [PubMed] [Google Scholar]
- Taylor A. M., Harnden D. G., Arlett C. F., Harcourt S. A., Lehmann A. R., Stevens S., Bridges B. A. Ataxia telangiectasia: a human mutation with abnormal radiation sensitivity. Nature. 1975 Dec 4;258(5534):427–429. doi: 10.1038/258427a0. [DOI] [PubMed] [Google Scholar]
- Thacker J., Chalk J., Ganesh A., North P. A mechanism for deletion formation in DNA by human cell extracts: the involvement of short sequence repeats. Nucleic Acids Res. 1992 Dec 11;20(23):6183–6188. doi: 10.1093/nar/20.23.6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacker J., Ganesh A. N. DNA-break repair, radioresistance of DNA synthesis, and camptothecin sensitivity in the radiation-sensitive irs mutants: comparisons to ataxia-telangiectasia cells. Mutat Res. 1990 Mar;235(2):49–58. doi: 10.1016/0921-8777(90)90057-c. [DOI] [PubMed] [Google Scholar]
- West C. M., Davidson S. E., Roberts S. A., Hunter R. D. Intrinsic radiosensitivity and prediction of patient response to radiotherapy for carcinoma of the cervix. Br J Cancer. 1993 Oct;68(4):819–823. doi: 10.1038/bjc.1993.434. [DOI] [PMC free article] [PubMed] [Google Scholar]


