Abstract
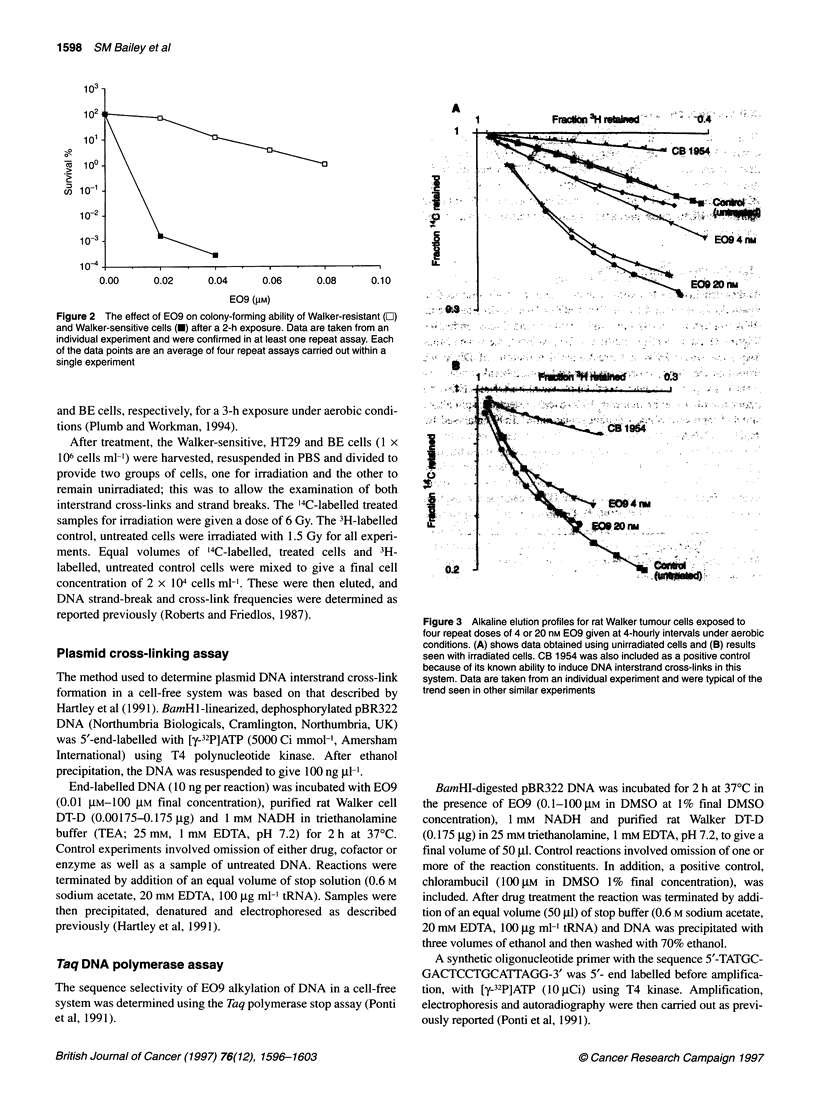
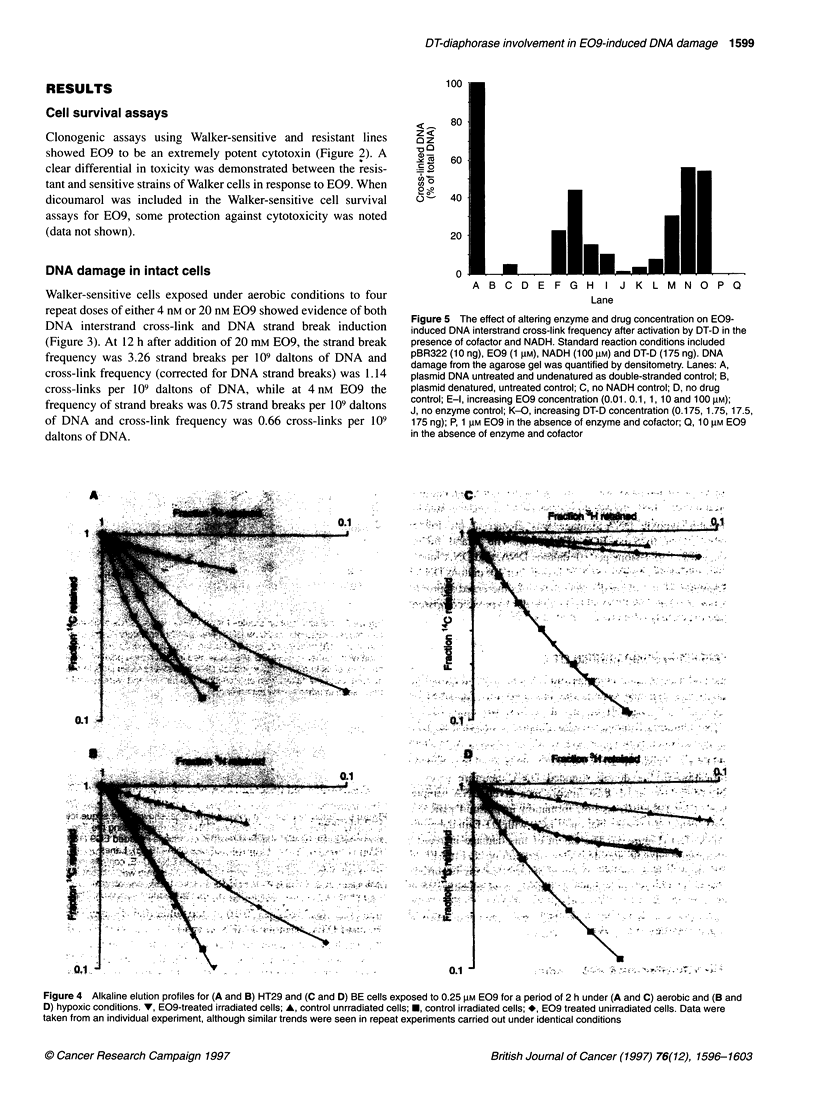
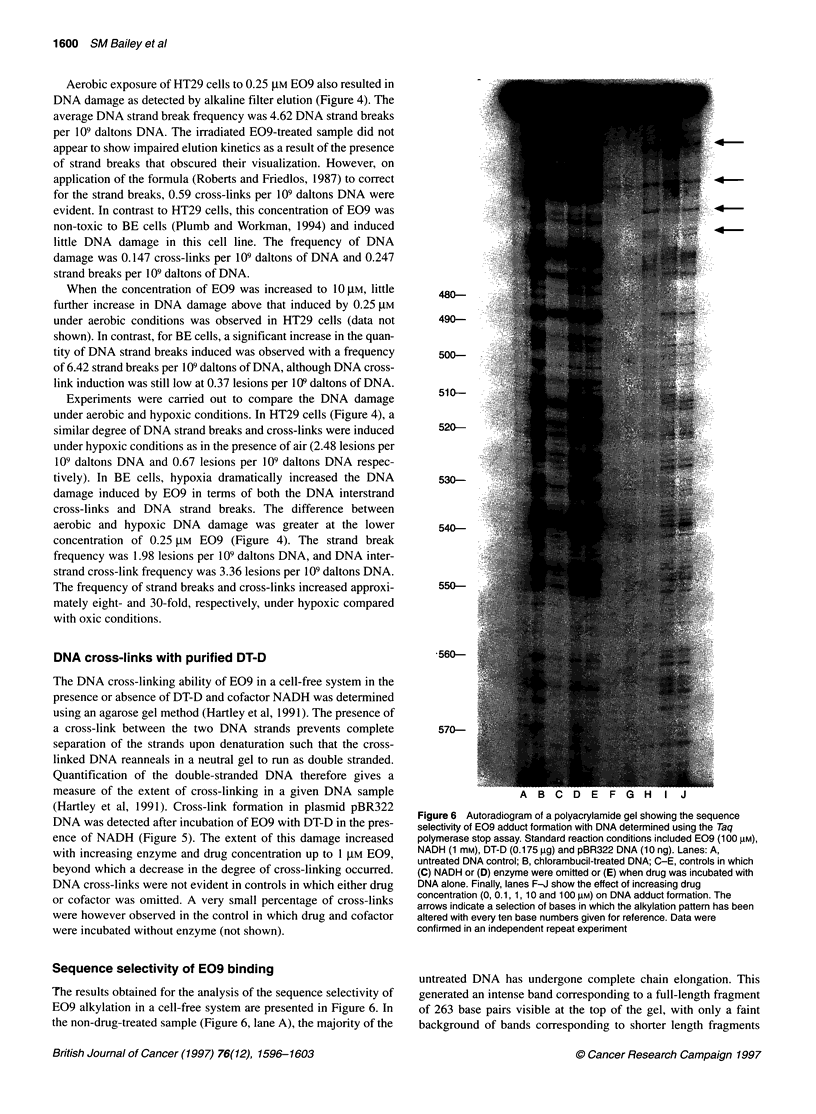
The chemistry of the mitomycin C-related drug indoloquinone EO9 would suggest that its mechanism of action is likely to involve DNA damage after reductive activation. The ability of this agent to induce DNA damage in intact cells has been examined using alkaline filter elution. After treatment with pharmacologically relevant concentrations of EO9, both DNA strand breaks and interstrand cross-links were detected in rat Walker tumour cells and human HT29 colon carcinoma cells. These cell lines express relatively high levels of DT-diaphorase (NAD(P)H: quinone acceptor oxidoreductase), which is believed to be involved in EO9 activation. The extent of DNA damage was increased by approximately 30-fold under hypoxia in BE colon carcinoma cells that express non-functional DT-diaphorase, but this dramatic hypoxia enhancement was not seen in HT-29 cells. These data are consistent with cytotoxicity studies that indicate that DT-diaphorase appears to be important in EO9 activation under aerobic conditions, but other enzymes may be more relevant under hypoxia. The involvement of DT-diaphorase in DNA damage induction was further investigated using cell-free assays. DNA cross-links were detectable in plasmid DNA co-incubated with EO9, cofactor and DT-diaphorase but not in the absence of this enzyme. In contrast, using a Taq polymerase stop assay, monofunctional alkylation was detected in plasmid DNA without metabolic activation, although the sequence selectivity was altered after reduction catalysed by DT-diaphorase.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey S. M., Suggett N., Walton M. I., Workman P. Structure-activity relationships for DT-diaphorase reduction of hypoxic cell directed agents: indoloquinones and diaziridinyl benzoquinones. Int J Radiat Oncol Biol Phys. 1992;22(4):649–653. doi: 10.1016/0360-3016(92)90496-5. [DOI] [PubMed] [Google Scholar]
- Chen S., Knox R., Lewis A. D., Friedlos F., Workman P., Deng P. S., Fung M., Ebenstein D., Wu K., Tsai T. M. Catalytic properties of NAD(P)H:quinone acceptor oxidoreductase: study involving mouse, rat, human, and mouse-rat chimeric enzymes. Mol Pharmacol. 1995 May;47(5):934–939. [PubMed] [Google Scholar]
- Collard J., Matthew A. M., Double J. A., Bibby M. C. EO9: relationship between DT-diaphorase levels and response in vitro and in vivo. Br J Cancer. 1995 Jun;71(6):1199–1203. doi: 10.1038/bjc.1995.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimmons S. A., Workman P., Grever M., Paull K., Camalier R., Lewis A. D. Reductase enzyme expression across the National Cancer Institute Tumor cell line panel: correlation with sensitivity to mitomycin C and EO9. J Natl Cancer Inst. 1996 Mar 6;88(5):259–269. doi: 10.1093/jnci/88.5.259. [DOI] [PubMed] [Google Scholar]
- Hartley J. A., Berardini M. D., Souhami R. L. An agarose gel method for the determination of DNA interstrand crosslinking applicable to the measurement of the rate of total and "second-arm" crosslink reactions. Anal Biochem. 1991 Feb 15;193(1):131–134. doi: 10.1016/0003-2697(91)90052-u. [DOI] [PubMed] [Google Scholar]
- Hendriks H. R., Pizao P. E., Berger D. P., Kooistra K. L., Bibby M. C., Boven E., Dreef-van der Meulen H. C., Henrar R. E., Fiebig H. H., Double J. A. EO9: a novel bioreductive alkylating indoloquinone with preferential solid tumour activity and lack of bone marrow toxicity in preclinical models. Eur J Cancer. 1993;29A(6):897–906. doi: 10.1016/s0959-8049(05)80434-4. [DOI] [PubMed] [Google Scholar]
- Knox R. J., Friedlos F., Jarman M., Roberts J. J. A new cytotoxic, DNA interstrand crosslinking agent, 5-(aziridin-1-yl)-4-hydroxylamino-2-nitrobenzamide, is formed from 5-(aziridin-1-yl)-2,4-dinitrobenzamide (CB 1954) by a nitroreductase enzyme in Walker carcinoma cells. Biochem Pharmacol. 1988 Dec 15;37(24):4661–4669. doi: 10.1016/0006-2952(88)90335-8. [DOI] [PubMed] [Google Scholar]
- Knox R. J., Lydall D. A., Friedlos F., Basham C., Rawlings C. J., Roberts J. J. The Walker 256 carcinoma: a cell type inherently sensitive only to those difunctional agents that can form DNA interstrand crosslinks. Mutat Res. 1991 Nov;255(3):227–240. doi: 10.1016/0921-8777(91)90026-l. [DOI] [PubMed] [Google Scholar]
- Kohn K. W., Hartley J. A., Mattes W. B. Mechanisms of DNA sequence selective alkylation of guanine-N7 positions by nitrogen mustards. Nucleic Acids Res. 1987 Dec 23;15(24):10531–10549. doi: 10.1093/nar/15.24.10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. S., Hartley J. A., Berardini M. D., Butler J., Siegel D., Ross D., Gibson N. W. Alteration in DNA cross-linking and sequence selectivity of a series of aziridinylbenzoquinones after enzymatic reduction by DT-diaphorase. Biochemistry. 1992 Mar 24;31(11):3019–3025. doi: 10.1021/bi00126a025. [DOI] [PubMed] [Google Scholar]
- Maliepaard M., Wolfs A., Groot S. E., de Mol N. J., Janssen L. H. Indoloquinone EO9: DNA interstrand cross-linking upon reduction by DT-diaphorase or xanthine oxidase. Br J Cancer. 1995 Apr;71(4):836–839. doi: 10.1038/bjc.1995.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattes W. B., Hartley J. A., Kohn K. W. DNA sequence selectivity of guanine-N7 alkylation by nitrogen mustards. Nucleic Acids Res. 1986 Apr 11;14(7):2971–2987. doi: 10.1093/nar/14.7.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlidis N., Hanauske A. R., Gamucci T., Smyth J., Lehnert M., te Velde A., Lan J., Verweij J. A randomized phase II study with two schedules of the novel indoloquinone EO9 in non-small-cell lung cancer: a study of the EORTC Early Clinical Studies Group (ECSG). Ann Oncol. 1996 Jul;7(5):529–531. doi: 10.1093/oxfordjournals.annonc.a010645. [DOI] [PubMed] [Google Scholar]
- Phillips R. M. Bioreductive activation of a series of analogues of 5-aziridinyl-3-hydroxymethyl-1-methyl-2-[1H-indole-4, 7-dione] prop-beta-en-alpha-ol (EO9) by human DT-diaphorase. Biochem Pharmacol. 1996 Dec 13;52(11):1711–1718. doi: 10.1016/s0006-2952(96)00521-7. [DOI] [PubMed] [Google Scholar]
- Plumb J. A., Gerritsen M., Milroy R., Thomson P., Workman P. Relative importance of DT-diaphorase and hypoxia in the bioactivation of EO9 by human lung tumor cell lines. Int J Radiat Oncol Biol Phys. 1994 May 15;29(2):295–299. doi: 10.1016/0360-3016(94)90278-x. [DOI] [PubMed] [Google Scholar]
- Plumb J. A., Gerritsen M., Workman P. DT-diaphorase protects cells from the hypoxic cytotoxicity of indoloquinone EO9. Br J Cancer. 1994 Dec;70(6):1136–1143. doi: 10.1038/bjc.1994.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumb J. A., Workman P. Unusually marked hypoxic sensitization to indoloquinone EO9 and mitomycin C in a human colon-tumour cell line that lacks DT-diaphorase activity. Int J Cancer. 1994 Jan 2;56(1):134–139. doi: 10.1002/ijc.2910560124. [DOI] [PubMed] [Google Scholar]
- Roberts J. J., Friedlos F. Quantitative estimation of cisplatin-induced DNA interstrand cross-links and their repair in mammalian cells: relationship to toxicity. Pharmacol Ther. 1987;34(2):215–246. doi: 10.1016/0163-7258(87)90012-x. [DOI] [PubMed] [Google Scholar]
- Roberts J. J., Marchbank T., Kotsaki-Kovatsi V. P., Boland M. P., Friedlos F., Knox R. J. Caffeine, aminoimidazolecarboxamide and dicoumarol, inhibitors of NAD(P)H dehydrogenase (quinone) (DT diaphorase), prevent both the cytotoxicity and DNA interstrand crosslinking produced by 5-(aziridin-1-yl)-2,4-dinitrobenzamide (CB 1954) in Walker cells. Biochem Pharmacol. 1989 Nov 15;38(22):4137–4143. doi: 10.1016/0006-2952(89)90695-3. [DOI] [PubMed] [Google Scholar]
- Robertson N., Haigh A., Adams G. E., Stratford I. J. Factors affecting sensitivity to EO9 in rodent and human tumour cells in vitro: DT-diaphorase activity and hypoxia. Eur J Cancer. 1994;30A(7):1013–1019. doi: 10.1016/0959-8049(94)90134-1. [DOI] [PubMed] [Google Scholar]
- Rosenoer V. M., Mitchley B. C., Roe F. J., Connors T. A. Walker carcinosarcoma 256 in study of anticancer agents. I. Method for simultaneous assessment of therapeutic value and toxicity. Cancer Res. 1966 Aug;26(8 Pt 2):937–941. [PubMed] [Google Scholar]
- Schellens J. H., Planting A. S., van Acker B. A., Loos W. J., de Boer-Dennert M., van der Burg M. E., Koier I., Krediet R. T., Stoter G., Verweij J. Phase I and pharmacologic study of the novel indoloquinone bioreductive alkylating cytotoxic drug E09. J Natl Cancer Inst. 1994 Jun 15;86(12):906–912. doi: 10.1093/jnci/86.12.906. [DOI] [PubMed] [Google Scholar]
- Siegel D., Beall H., Senekowitsch C., Kasai M., Arai H., Gibson N. W., Ross D. Bioreductive activation of mitomycin C by DT-diaphorase. Biochemistry. 1992 Sep 1;31(34):7879–7885. doi: 10.1021/bi00149a019. [DOI] [PubMed] [Google Scholar]
- Siegel D., Gibson N. W., Preusch P. C., Ross D. Metabolism of diaziquone by NAD(P)H:(quinone acceptor) oxidoreductase (DT-diaphorase): role in diaziquone-induced DNA damage and cytotoxicity in human colon carcinoma cells. Cancer Res. 1990 Nov 15;50(22):7293–7300. [PubMed] [Google Scholar]
- Siegel D., Gibson N. W., Preusch P. C., Ross D. Metabolism of mitomycin C by DT-diaphorase: role in mitomycin C-induced DNA damage and cytotoxicity in human colon carcinoma cells. Cancer Res. 1990 Dec 1;50(23):7483–7489. [PubMed] [Google Scholar]
- Smitskamp-Wilms E., Peters G. J., Pinedo H. M., van Ark-Otte J., Giaccone G. Chemosensitivity to the indoloquinone EO9 is correlated with DT-diaphorase activity and its gene expression. Biochem Pharmacol. 1994 Apr 20;47(8):1325–1332. doi: 10.1016/0006-2952(94)90330-1. [DOI] [PubMed] [Google Scholar]
- Walton M. I., Bibby M. C., Double J. A., Plumb J. A., Workman P. DT-diaphorase activity correlates with sensitivity to the indoloquinone EO9 in mouse and human colon carcinomas. Eur J Cancer. 1992;28A(10):1597–1600. doi: 10.1016/0959-8049(92)90049-8. [DOI] [PubMed] [Google Scholar]
- Walton M. I., Smith P. J., Workman P. The role of NAD(P)H: quinone reductase (EC 1.6.99.2, DT-diaphorase) in the reductive bioactivation of the novel indoloquinone antitumor agent EO9. Cancer Commun. 1991 Jul;3(7):199–206. doi: 10.3727/095535491820873164. [DOI] [PubMed] [Google Scholar]
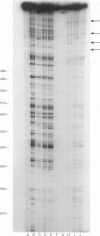
- Workman P. Enzyme-directed bioreductive drug development revisited: a commentary on recent progress and future prospects with emphasis on quinone anticancer agents and quinone metabolizing enzymes, particularly DT-diaphorase. Oncol Res. 1994;6(10-11):461–475. [PubMed] [Google Scholar]